tổ cal ate the In the sample. 6. Calculate the molarity of a stock ammonia solution that has a mass percent of 28.0 (w/w) and a specific gravity of 0.90. How would you prepare a 0.15 M NH3 solution in 250 mL from this stock?
tổ cal ate the In the sample. 6. Calculate the molarity of a stock ammonia solution that has a mass percent of 28.0 (w/w) and a specific gravity of 0.90. How would you prepare a 0.15 M NH3 solution in 250 mL from this stock?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter16: Reactions Between Acids And Bases
Section: Chapter Questions
Problem 16.93QE
Related questions
Question
Answer 6 question and part 2 both
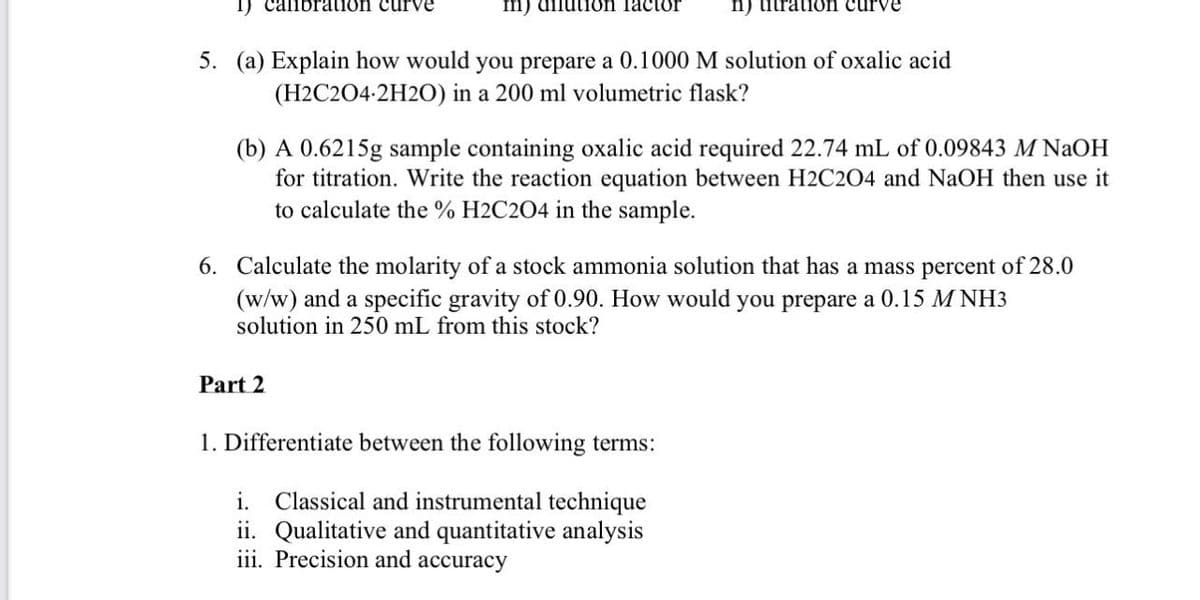
Transcribed Image Text:I) callbration curve
m) dilution factor
n) titratioön curve
5. (a) Explain how would you prepare a 0.1000 M solution of oxalic acid
(H2C204-2H20) in a 200 ml volumetric flask?
(b) A 0.6215g sample containing oxalic acid required 22.74 mL of 0.09843 M NaOH
for titration. Write the reaction equation between H2C204 and NaOH then use it
to calculate the % H2C2O4 in the sample.
6. Calculate the molarity of a stock ammonia solution that has a mass percent of 28.0
(w/w) and a specific gravity of 0.90. How would you prepare a 0.15 M NH3
solution in 250 mL from this stock?
Part 2
1. Differentiate between the following terms:
i. Classical and instrumental technique
ii. Qualitative and quantitative analysis
iii. Precision and accuracy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

