To the right is a representation of 50 atoms of a fictitious element called westmontium (Wt). The red spheres represent Wt-296, the blue spheres Wi-297. and the green spheres Wt-298. The mass of each Wt isotope is measured relative to C-12 and tabulated here. Use the mass of C-12 to convert each of the masses to amu and calculate the atomic mass of Wt. Isotope Mass Wt-296 24.6630 x Mass("C) Wt-297 24.7490 x Mass("C) Wt-298 24.8312 x Mass("C)
To the right is a representation of 50 atoms of a fictitious element called westmontium (Wt). The red spheres represent Wt-296, the blue spheres Wi-297. and the green spheres Wt-298. The mass of each Wt isotope is measured relative to C-12 and tabulated here. Use the mass of C-12 to convert each of the masses to amu and calculate the atomic mass of Wt. Isotope Mass Wt-296 24.6630 x Mass("C) Wt-297 24.7490 x Mass("C) Wt-298 24.8312 x Mass("C)
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter2: Atoms
Section: Chapter Questions
Problem 2.103P: 2-103 The element silver has two naturally occurring isotopes: 109Ag and 107Ag with a mass of...
Related questions
Question
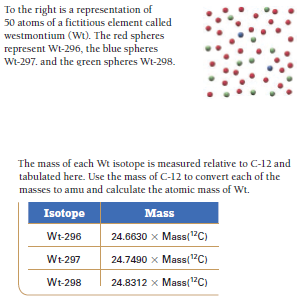
Transcribed Image Text:To the right is a representation of
50 atoms of a fictitious element called
westmontium (Wt). The red spheres
represent Wt-296, the blue spheres
Wi-297. and the green spheres Wt-298.
The mass of each Wt isotope is measured relative to C-12 and
tabulated here. Use the mass of C-12 to convert each of the
masses to amu and calculate the atomic mass of Wt.
Isotope
Mass
Wt-296
24.6630 x Mass("C)
Wt-297
24.7490 x Mass("C)
Wt-298
24.8312 x Mass("C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning