TRIAL 2 Determining the Specific Heat of Metal Specimen Metal Specimen 2 Mass of calorimeter, m: 125.0 g Mass of calorimeter with water 227.0 g Mass of water, Miw 102g Mass of metal specimen, mm 51.0 g Initial temperature of metal, tam 100'C Initial temperature of calorimeter, tc 25.0°C Initial temperature of water, tow 25.0°C Final temperature of the mixture, tmix 31.0°C Specific Heat of Water ow 1.0000 cal/gC Specific Heat of calorimeter e 0.2174 cal/gC Experimental specific heat of metal, Cm calgC Actual specific heat of metal, C 0.2174 cal/gC Percentage Error
TRIAL 2 Determining the Specific Heat of Metal Specimen Metal Specimen 2 Mass of calorimeter, m: 125.0 g Mass of calorimeter with water 227.0 g Mass of water, Miw 102g Mass of metal specimen, mm 51.0 g Initial temperature of metal, tam 100'C Initial temperature of calorimeter, tc 25.0°C Initial temperature of water, tow 25.0°C Final temperature of the mixture, tmix 31.0°C Specific Heat of Water ow 1.0000 cal/gC Specific Heat of calorimeter e 0.2174 cal/gC Experimental specific heat of metal, Cm calgC Actual specific heat of metal, C 0.2174 cal/gC Percentage Error
Physics for Scientists and Engineers, Technology Update (No access codes included)
9th Edition
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter20: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 20.10OQ: A 100-g piece of copper, initially at 95.0C, is dropped into 200 g of water contained in a 280-g...
Related questions
Question
with the aluminum as metal, find the Experimental specific heat of metal, cm and the percentage error with clear solution.
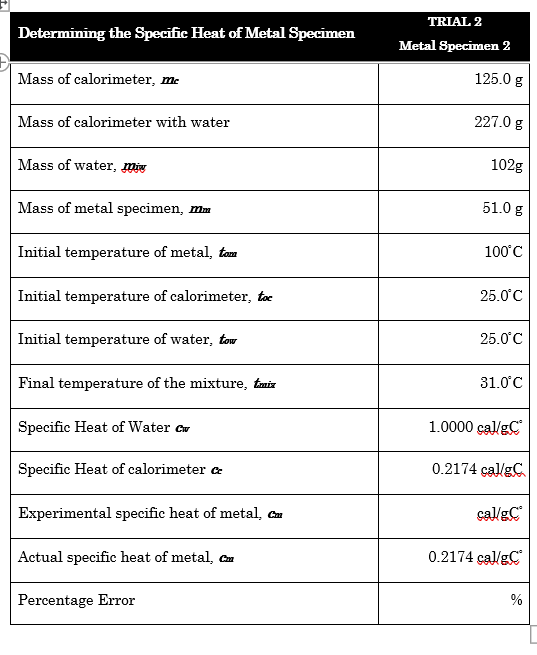
Transcribed Image Text:TRIAL 2
Determining the Specific Heat of Metal Specimen
Metal Specimen 2
Mass of calorimeter, me
125.0 g
Mass of calorimeter with water
227.0 g
Mass of water, miw
102g
Mass of metal specimen, mm
51.0 g
Initial temperature of metal, tam
100°C
Initial temperature of calorimeter, toc
25.0°C
Initial temperature of water, tow
25.0°C
Final temperature of the mixture, tmix
31.0°C
Specific Heat of Water Cw
1.0000 calgC
Specific Heat of calorimeter c
0.2174 cal/gC
Experimental specific heat of metal, Cam
cal/gC
Actual specific heat of metal, Cm
0.2174 cal/gC
Percentage Error
%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College



An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning