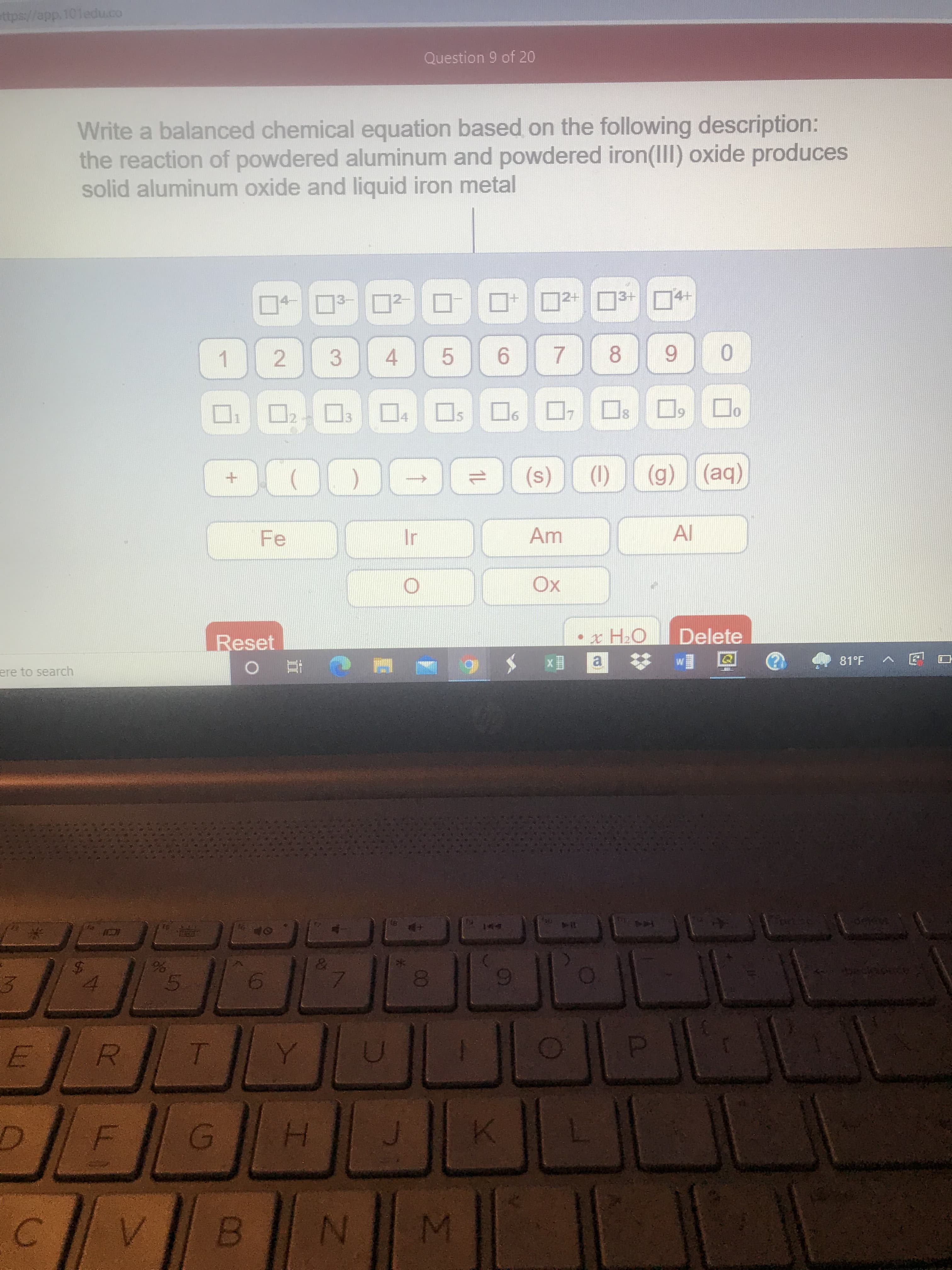
ttps://app.101edu.co Question 9 of 20 Write a balanced chemical equation based on the following description: the reaction of powdered aluminum and powdered iron(III) oxide produces solid aluminum oxide and liquid iron metal
ttps://app.101edu.co Question 9 of 20 Write a balanced chemical equation based on the following description: the reaction of powdered aluminum and powdered iron(III) oxide produces solid aluminum oxide and liquid iron metal
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter6: Chemical Reactions: An Introduction
Section: Chapter Questions
Problem 50AP: The Hall process is an important method by which pure aluminum is prepared from its oxide (alumina,...
Related questions
Question
100%
Transcribed Image Text:C.
24
ere to search
B.
G)
5.
H.
ttps://app.101edu.co
Reset
solid aluminum oxide and liquid iron metal
the reaction of powdered aluminum and powdered iron(III) oxide produces
Write a balanced chemical equation based on the following description:
Fe
1 2
C直 0
2.
3.
4 5
目x
a.
(s)
9.
Question 9 of 20
(1)
7.
2+ 3+
Delete
口日日
(g) (aq)
8.
Al
(be)
81°F
口 回v
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
