Two closed tanks are connected to each other by a valve. The first tank contains oxygen (02, m 2.1 kg, 7 = 132 p=4 bar) and the other carbon dioxide (CO₂, m= 2.3 kg, T = 27 °C, p= 1.5 bar). When the valve is opened, the gases are allowed to mix. When the mixture reaches equilibrium, the temperature of the mixture is 78 °C. The gases can be assumed to be ideal gases. Calculate m3 (two decimal accuracy) 1) Total volume of the tanks 2) Final pressure of the mixture 3) Molar fraction of oxygen in the mixture 4) Molar fraction of carbon dioxide in the mixture 5) Partial pressure of oxygen in the mixture 6) Partial pressure of carbon dioxide in the mixture kPa (zero decimal accuracy) % (zero decimal accuracy) % (zero decimal accuracy) kPa (zero decimal accuracy) kPa (zero decimal accuracy)
Two closed tanks are connected to each other by a valve. The first tank contains oxygen (02, m 2.1 kg, 7 = 132 p=4 bar) and the other carbon dioxide (CO₂, m= 2.3 kg, T = 27 °C, p= 1.5 bar). When the valve is opened, the gases are allowed to mix. When the mixture reaches equilibrium, the temperature of the mixture is 78 °C. The gases can be assumed to be ideal gases. Calculate m3 (two decimal accuracy) 1) Total volume of the tanks 2) Final pressure of the mixture 3) Molar fraction of oxygen in the mixture 4) Molar fraction of carbon dioxide in the mixture 5) Partial pressure of oxygen in the mixture 6) Partial pressure of carbon dioxide in the mixture kPa (zero decimal accuracy) % (zero decimal accuracy) % (zero decimal accuracy) kPa (zero decimal accuracy) kPa (zero decimal accuracy)
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
The answers from 1 to 6 are:
1.42
242
56%
44%
136kPa
107kPa
Please solve for questions 7 8 9
Please do not rely too much on chatgpt, because its answer may be wrong. Please consider it carefully and give your own answer. You can borrow ideas from gpt, but please do not believe its answer.
Very very grateful!
Please do not rely too much on chatgpt, because its answer may be wrong. Please consider it carefully and give your own answer. You can borrow ideas from gpt, but please do not believe its answer.
Very very grateful!
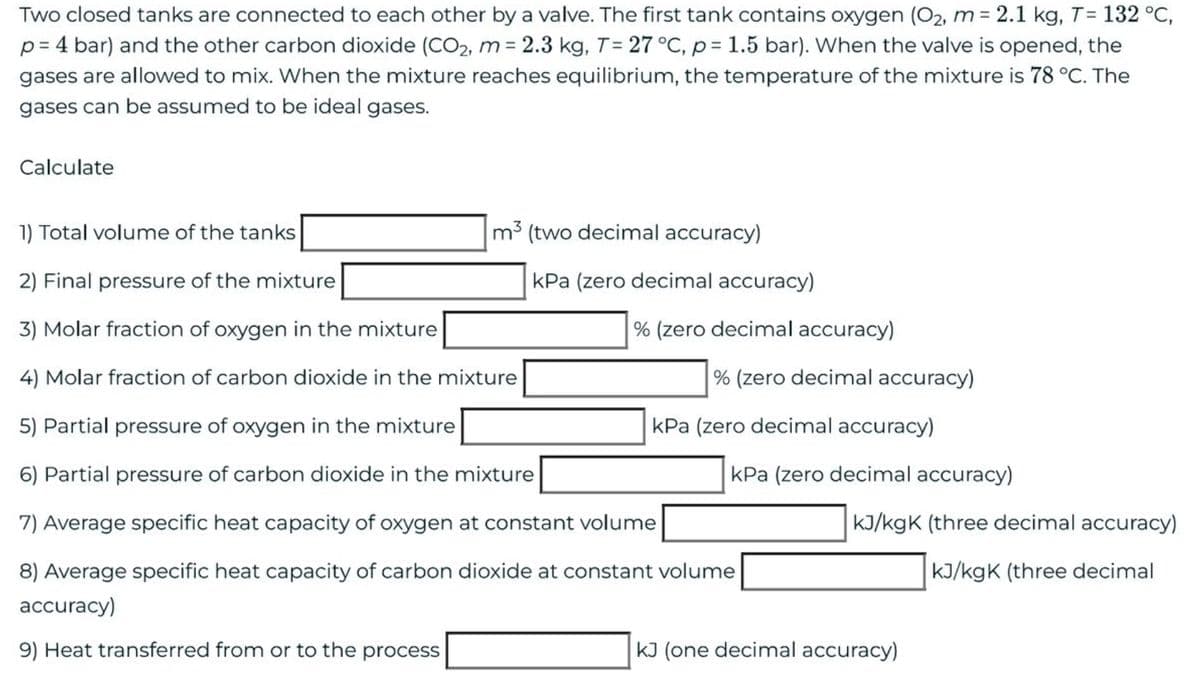
Transcribed Image Text:Two closed tanks are connected to each other by a valve. The first tank contains oxygen (O₂, m= 2.1 kg, T= 132 °C,
p=4 bar) and the other carbon dioxide (CO₂, m= 2.3 kg, T = 27 °C, p = 1.5 bar). When the valve is opened, the
gases are allowed to mix. When the mixture reaches equilibrium, the temperature of the mixture is 78 °C. The
gases can be assumed to be ideal gases.
Calculate
m3 (two decimal accuracy)
1) Total volume of the tanks
2) Final pressure of the mixture
3) Molar fraction of oxygen in the mixture
4) Molar fraction of carbon dioxide in the mixture
5) Partial pressure of oxygen in the mixture
6) Partial pressure of carbon dioxide in the mixture
7) Average specific heat capacity of oxygen at constant volume
8) Average specific heat capacity of carbon dioxide at constant volume
accuracy)
9) Heat transferred from or to the process
kPa (zero decimal accuracy)
% (zero decimal accuracy)
% (zero decimal accuracy)
kPa (zero decimal accuracy)
kPa (zero decimal accuracy)
kJ/kgK (three decimal accuracy)
kJ/kgK (three decimal
kJ (one decimal accuracy)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step 1: To determine the volume of tank, the final pressure of mixture, molar fraction and partial pressure.
VIEWStep 2: Calculate the total volume of the tanks and the final pressure of the mixture.
VIEWStep 3: Calculate the mole of oxygen and carbon dioxide and total number of moles.
VIEWStep 4: Calculate the molar fraction of oxygen and the molar fraction of carbon dioxide
VIEWSolution
VIEWStep by step
Solved in 5 steps with 22 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY