Two Insulated cylinders A and B with volumes VA 1.2 m³ and VB 6.4 m³ contain chlorine gas at different pressures and temperatures. The cylinders are insulated (no heat is lost to or gained from the outside) and connected by a valve. Initially, the valve is closed and the gas in the two cylinders has the following values: PA 4.0 x 105 N/m2, TA - 250 K, PB - 2.5 x 105 N/m², Tg = 580 K. The valve is opened to allow the contents in the two cylinders to mix until the pressure equalizes. valve (a) Assuming there is no change in the temperatures of the containers themselves, determine the final temperature of the gas in the two cylinders. The atomic mass of chlorine gas is 35.4527 u. (b) Determine the final pressure. N/m2
Two Insulated cylinders A and B with volumes VA 1.2 m³ and VB 6.4 m³ contain chlorine gas at different pressures and temperatures. The cylinders are insulated (no heat is lost to or gained from the outside) and connected by a valve. Initially, the valve is closed and the gas in the two cylinders has the following values: PA 4.0 x 105 N/m2, TA - 250 K, PB - 2.5 x 105 N/m², Tg = 580 K. The valve is opened to allow the contents in the two cylinders to mix until the pressure equalizes. valve (a) Assuming there is no change in the temperatures of the containers themselves, determine the final temperature of the gas in the two cylinders. The atomic mass of chlorine gas is 35.4527 u. (b) Determine the final pressure. N/m2
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter3: Transient Heat Conduction
Section: Chapter Questions
Problem 3.12P
Related questions
Question
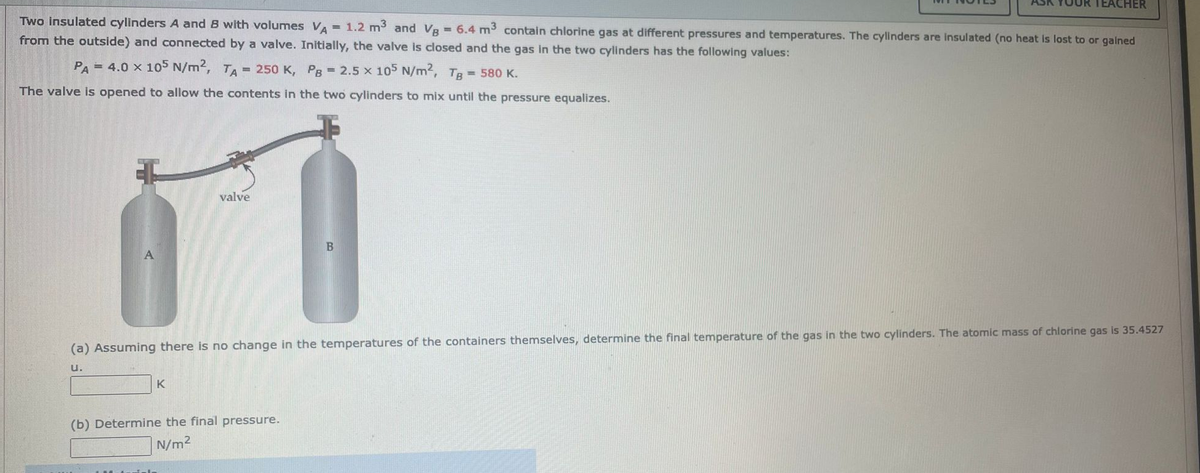
Transcribed Image Text:Two insulated cylinders A and B with volumes VA = 1.2 m³ and VB = 6.4 m³ contain chlorine gas at different pressures and temperatures. The cylinders are insulated (no heat is lost to or gained
from the outside) and connected by a valve. Initially, the valve is closed and the gas in the two cylinders has the following values:
PA= 4.0 x 105 N/m2, TA= 250 K, PB = 2.5 x 105 N/m², Tg = 580 K.
The valve is opened to allow the contents in the two cylinders to mix until the pressure equalizes.
valve
K
TEACHER
(a) Assuming there is no change in the temperatures of the containers themselves, determine the final temperature of the gas in the two cylinders. The atomic mass of chlorine gas is 35.4527
U.
(b) Determine the final pressure.
N/m²
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning