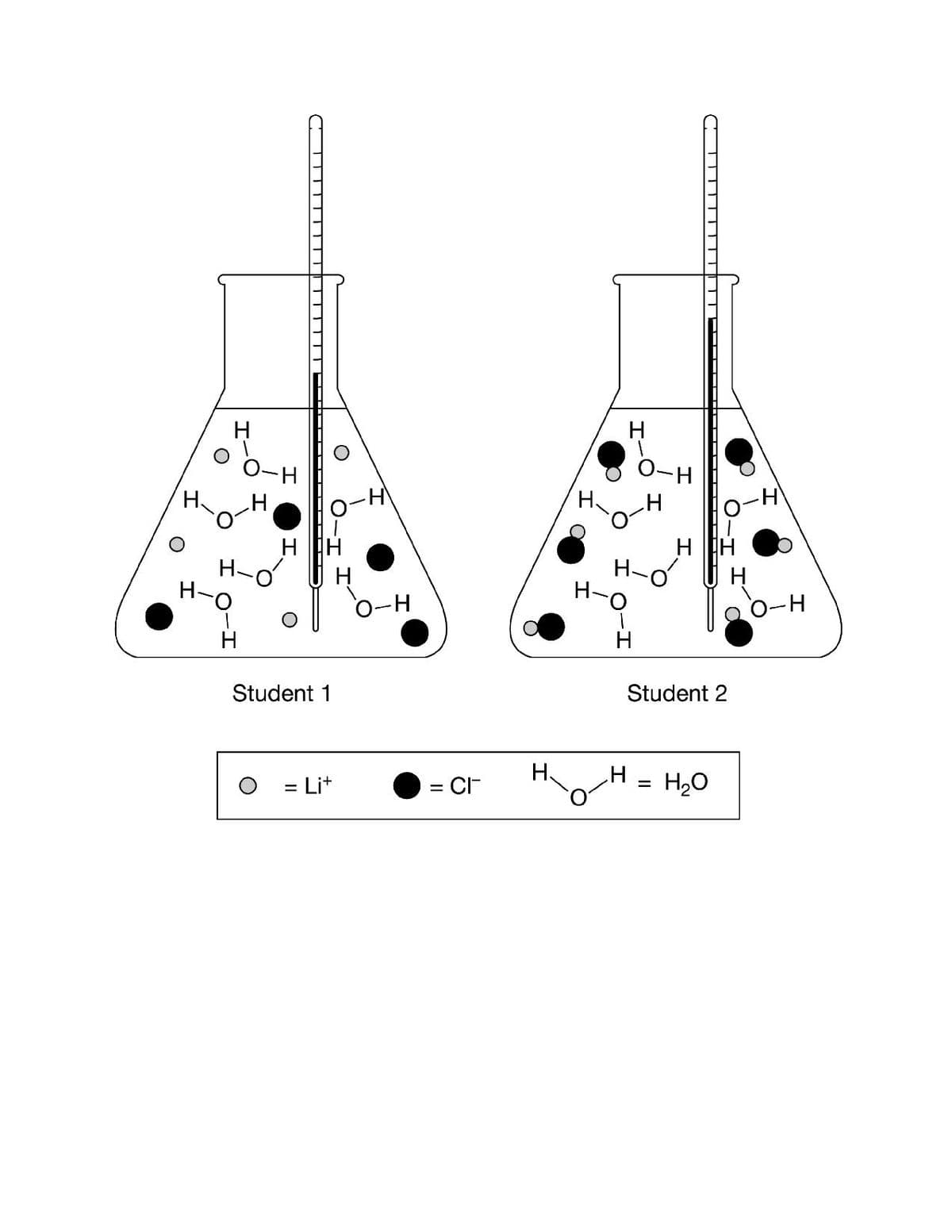
Two students prepared aqueous solutions of LiCl and measured the properties, as shown in the table above. Both students observed that the solid LiCl readily dissolved in H2O. The students drew particle diagrams to explain the changes in the enthalpy and entropy of dissolution for LiCl based on their results and observations.
Two students prepared aqueous solutions of LiCl and measured the properties, as shown in the table above. Both students observed that the solid LiCl readily dissolved in H2O. The students drew particle diagrams to explain the changes in the enthalpy and entropy of dissolution for LiCl based on their results and observations.
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter2: Measurements And Calculations
Section: Chapter Questions
Problem 104AP: Convert 45 mi/h to m/s, showing how the units cancel appropriately.
Related questions
Question
Two students prepared aqueous solutions of LiCl and measured the properties, as shown in the table above. Both students observed that the solid LiCl readily dissolved in H2O. The students drew particle diagrams to explain the changes in the enthalpy and entropy of dissolution for LiCl based on their results and observations. Based on this information, the better particle diagram was drawn by which student, and why is that diagram more accurate? (see attached image)
a.) The better particle diagram was drawn by Student 1 because when LiCl dissolves in water, it dissociates into Li+ and Cl− ions causing an increase in entropy.
b.) The better particle diagram was drawn by Student 1 because when LiCl dissolves in water, it dissociates into Li+and Cl−ions indicating that the dissolution of LiCl is exothermic.
c.) The better particle diagram was drawn by Student 2 because it shows that LiCl does not dissociate, resulting in a decrease in entropy for the dissolution of LiCl.
d.) The better particle diagram was drawn by Student 2 because it shows that LiCl does not dissociate, resulting in an endothermic process for the dissolution of LiCl.
Transcribed Image Text:H
0-H
HH
H-o
H-0
H-o
H-O
H
O-H
0-H
H
H
Student 1
Student 2
Н.
= Lit
= CI-
H20
%D
C II|- TT ---T--
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning