Un knawn # rial Mass of metit ( $3.25s tam Pera h he oFme ting uter mass of Ca letimetar A4:5 3S12 9) 62 5729 Mass af alenine ter Water a 「ちろ.443 mass f waterY2 Thi tal Tompera tule f Water in CalahineT Ce maximumof ter in Calorine TeY cED Te mperatuK ng Hart 8anes by Gh791 Tan Perature Chaage of nele NTCC SPecific hent Signature Date Witness Da Morton Publishing Chemistry Laboratory Notebook Note: Insert back cover flap under copy sheet beform
Un knawn # rial Mass of metit ( $3.25s tam Pera h he oFme ting uter mass of Ca letimetar A4:5 3S12 9) 62 5729 Mass af alenine ter Water a 「ちろ.443 mass f waterY2 Thi tal Tompera tule f Water in CalahineT Ce maximumof ter in Calorine TeY cED Te mperatuK ng Hart 8anes by Gh791 Tan Perature Chaage of nele NTCC SPecific hent Signature Date Witness Da Morton Publishing Chemistry Laboratory Notebook Note: Insert back cover flap under copy sheet beform
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter6: An Introduction To Spectrometric Methods
Section: Chapter Questions
Problem 6.19QAP
Related questions
Question
Average specific heat of the metal (J/g*°C)_______________
Questions
1.You assumed that the initial temperature of the metal sample was the same as that of the boiling water. If the metal sample was actually at a lower temperature than the boiling water, how would your value for the specific heat be affected?
2.When a student chemist transferred the metal to the calorimeter, some water splashed outof the calorimeter. How will this technique error affect the calculated specific heat of the
metal?
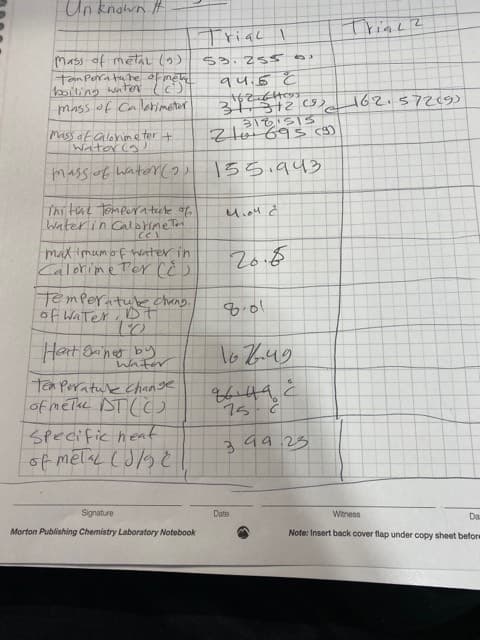
Transcribed Image Text:Un knawn #
rial
Mass of metit (
$3.25s
tam Pera h he oFme
ting uter
mass of Ca letimetar
A4:5
3S12 9) 62 5729
Mass af alenine ter
Water a
「ちろ.443
mass f waterY2
Thi tal Tompera tule f
Water in CalahineT
Ce
maximumof ter in
Calorine TeY cED
Te mperatuK ng
Hart 8anes by
Gh791
Tan Perature Chaage
of nele NTCC
SPecific hent
Signature
Date
Witness
Da
Morton Publishing Chemistry Laboratory Notebook
Note: Insert back cover flap under copy sheet beform
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning