Use Charles's law to complete the table. (Assume pressure and number of moles of gas to be constant.) Part V1 T1 V2 T2 A 115 L 10.6 °C 120.7 °C В 134 K 177 L 314 K C 2.02 L 15.0 °C 2.26 L 15.9 cm3 18.4 cm3 11.4 °C
Use Charles's law to complete the table. (Assume pressure and number of moles of gas to be constant.) Part V1 T1 V2 T2 A 115 L 10.6 °C 120.7 °C В 134 K 177 L 314 K C 2.02 L 15.0 °C 2.26 L 15.9 cm3 18.4 cm3 11.4 °C
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter7: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 7.37EP
Related questions
Question
100%
1. Complete the first row of the table.
Express the volume in liters to three significant figures.
2. Complete the second row of the table.
Express the volume in liters to three significant figures.
3. Complete the third row of the table.
Express the temperature in degrees Celsius to three significant figures.
4. Complete the fourth row of the table.
Express the temperature in degrees Celsius to three significant figures.
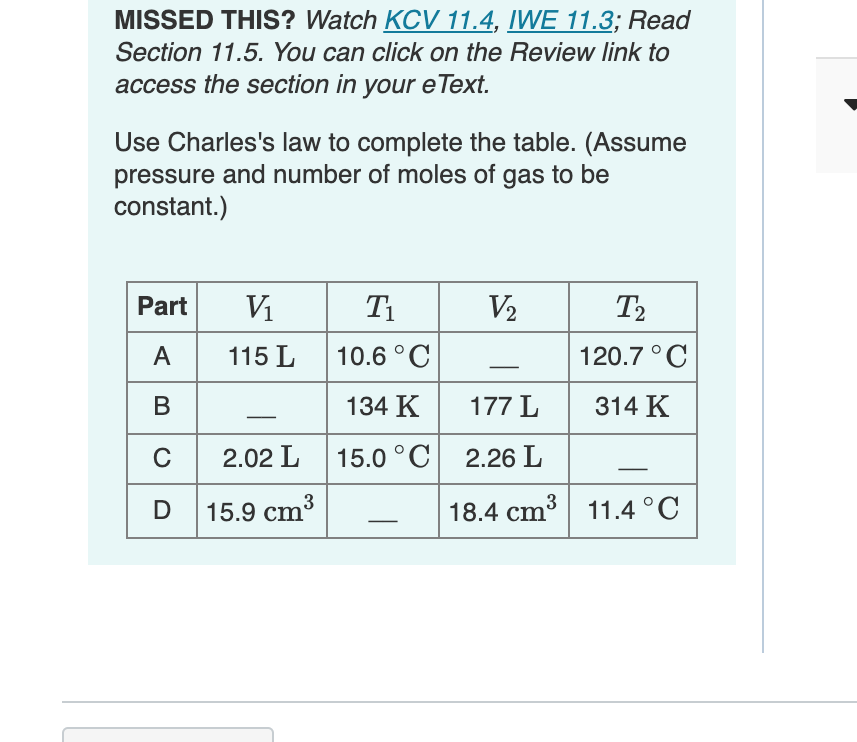
Transcribed Image Text:MISSED THIS? Watch KCV 11.4, IWE 11.3; Read
Section 11.5. You can click on the Review link to
access the section in your e Text.
Use Charles's law to complete the table. (Assume
pressure and number of moles of gas to be
constant.)
Part
Vi
T1
V2
T2
A
115 L
10.6 °C
120.7 °C
В
134 K
177 L
314 K
C 2.02 L
15.0 °C
2.26 L
D
15.9 cm3
18.4 cm3 11.4°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER