Use standard reduction potentials to calculate the standard free energy change in kJ for the reaction: 2Cu"(aq) + 21(aq) 2Ca (aq) + I(0) Answer: kJ K for this reaction would be than one greater
Use standard reduction potentials to calculate the standard free energy change in kJ for the reaction: 2Cu"(aq) + 21(aq) 2Ca (aq) + I(0) Answer: kJ K for this reaction would be than one greater
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter19: Principles Of Chemical Reactivity: Electron Transfer Reactions
Section19.9: Corrosion: Redox Reactions In The Environment
Problem 2.4ACP: The overall reaction for the production of Cu(OH)2 from Cu in oxygenated water can be broken into...
Related questions
Question
6
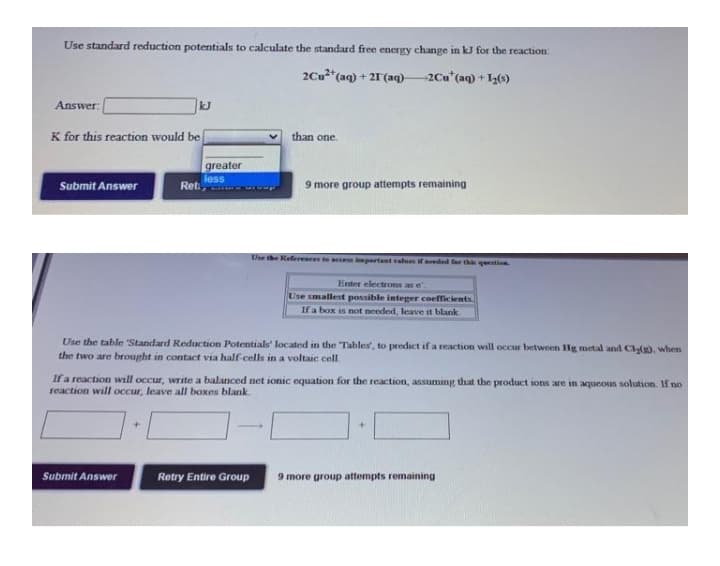
Transcribed Image Text:Use standard reduction potentials to calculate the standard free energy change in kJ for the reaction
2Cu*(aq) + 2T(aq)–2Cu*(aq) + 1,(0)
Answer:
kJ
K for this reaction would be
than one
greater
Submit Answer
less
Ret
9 more group attempts remaining
Uhe the References to acempartant valus if eeded fer thi qestin
Enter electrons as e.
Use smallest possible integer coefficients
If a box is not needed, leave it blank
Use the table 'Standard Reduction Potentials' located in the Tables, to predict if a reaction will occur between Hg metal and Cl(). when
the two are brought in contact via half-cells in a voltaic cell
If a reaction will occur, write a balanced net ionic equation for the reaction, assuming that the product ions are in aqueoun solution. If no
reaction will oCcur, leave all boxes blank.
Submit Answer
Retry Entire Group
9 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning