Use the Debye-Hückel equation to calculate the activity coefficient of each ion at the given ionic strength in an aqueous solution at 25 °C. Pb2+ in a solution where μ = 0.0611 M YPb²+ = PO3 in a solution where μ = 0.0483 M YPO- = > TOOLS X10% Ion Pb2+ Mg2+ Zn²+ Cro Cr³+ PO Zr¹ + Ce4+ Ion size (a, nm) 0.450 0.800 0.600 0.400 0.900 0.400 1.100 1.100
Use the Debye-Hückel equation to calculate the activity coefficient of each ion at the given ionic strength in an aqueous solution at 25 °C. Pb2+ in a solution where μ = 0.0611 M YPb²+ = PO3 in a solution where μ = 0.0483 M YPO- = > TOOLS X10% Ion Pb2+ Mg2+ Zn²+ Cro Cr³+ PO Zr¹ + Ce4+ Ion size (a, nm) 0.450 0.800 0.600 0.400 0.900 0.400 1.100 1.100
Chapter10: Effect Of Electrolytes On Chemical Equilibria
Section: Chapter Questions
Problem 10.8QAP
Related questions
Question
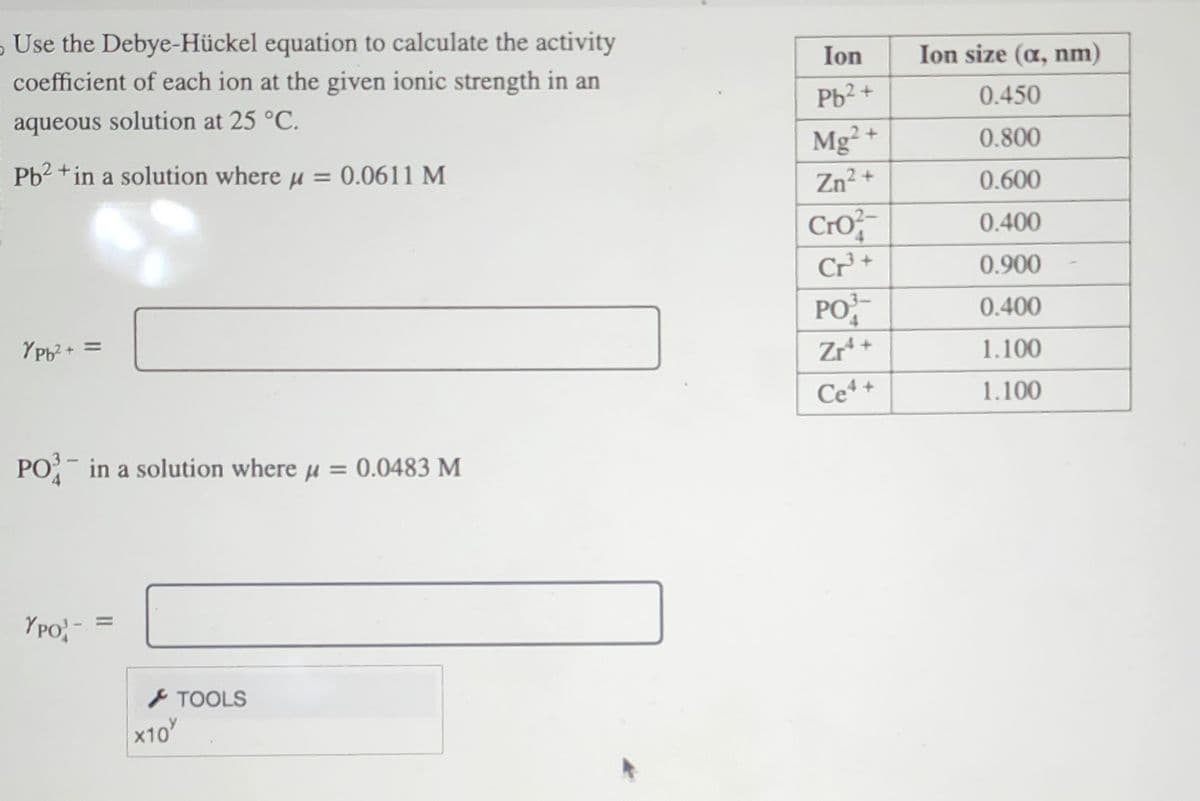
Transcribed Image Text:Use the Debye-Hückel equation to calculate the activity
coefficient of each ion at the given ionic strength in an
aqueous solution at 25 °C.
Pb2+ in a solution where μ = 0.0611 M
YPb²+ =
PO3 in a solution where μ = 0.0483 M
YPO
x10
TOOLS
Ion
Pb²+
Mg2+
Zn²+
Cro
Cr³+
PO
Zr¹+
Ce4+
Ion size (a, nm)
0.450
0.800
0.600
0.400
0.900
0.400
1.100
1.100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

