Use the References to access important values if needed for this question. 2Bi + HXeO4+ 3H2O- —Хе + 2Bi(ОН)з+ ОН In the above redox reaction, use oxidation numbers to identify the element oxidized, the element reduced, the oxidizing agent and the reducing agent. name of the element name of the element oxidized: reduced: formula of the formula of the oxidizing agent: reducing agent:
Use the References to access important values if needed for this question. 2Bi + HXeO4+ 3H2O- —Хе + 2Bi(ОН)з+ ОН In the above redox reaction, use oxidation numbers to identify the element oxidized, the element reduced, the oxidizing agent and the reducing agent. name of the element name of the element oxidized: reduced: formula of the formula of the oxidizing agent: reducing agent:
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 17RORR: Specify which of the following equations represent oxidation reduction reactions, and indicate the...
Related questions
Question
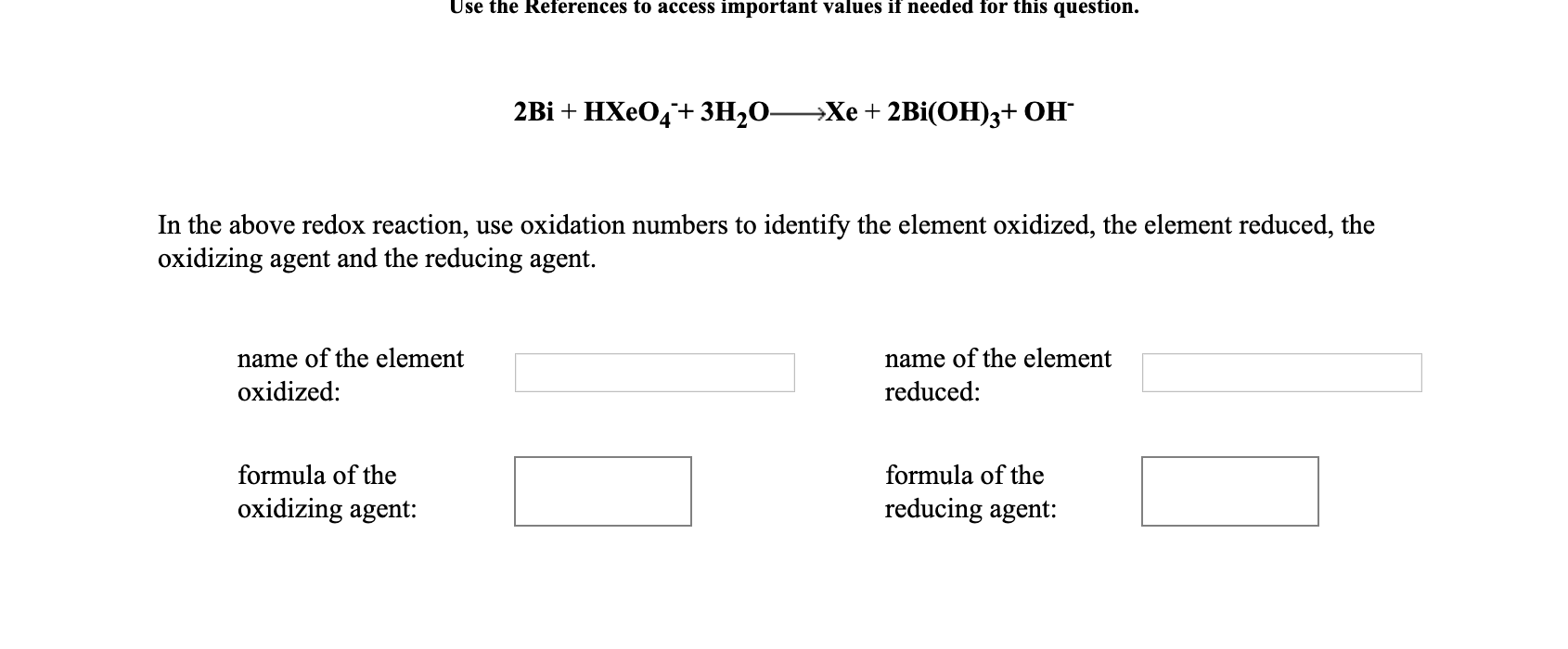
Transcribed Image Text:Use the References to access important values if needed for this question.
2Bi + HXeO4+ 3H2O-
—Хе + 2Bi(ОН)з+ ОН
In the above redox reaction, use oxidation numbers to identify the element oxidized, the element reduced, the
oxidizing agent and the reducing agent.
name of the element
name of the element
oxidized:
reduced:
formula of the
formula of the
oxidizing agent:
reducing agent:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning