Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG" for the following redox reaction. Round your answer to 3 significant digits. olo 2Cu* (aq)+2H,0 (1) → 2Cu** (aq) +H, (g) +2OH¯ (aq)
Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG" for the following redox reaction. Round your answer to 3 significant digits. olo 2Cu* (aq)+2H,0 (1) → 2Cu** (aq) +H, (g) +2OH¯ (aq)
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 150CP: Given the following two standard reduction potentials, solve for the standard reduction potential of...
Related questions
Question
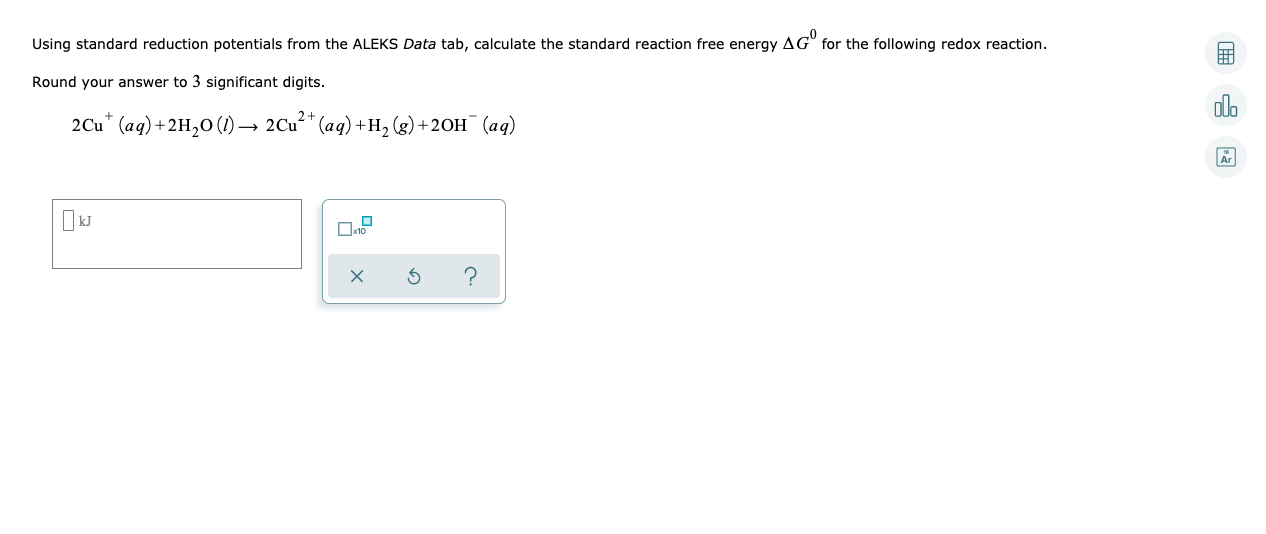
Transcribed Image Text:Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG" for the following redox reaction.
Round your answer to 3 significant digits.
olo
2Cu* (aq)+2H,0 (1) → 2Cu** (aq) +H, (g) +2OH¯ (aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning