a. Using the average atomic mass of carbon, calculate the mass (in amu) of 165 carbon atoms. amu b. Using the average atomic mass of potassium, calculate the mass (in amu) of 8.70 × 106 potassium atoms. amu c. Using the average atomic mass of lithium, calculate the mass (in amu) of 3.40 × 1023 of lithium atoms. amu
a. Using the average atomic mass of carbon, calculate the mass (in amu) of 165 carbon atoms. amu b. Using the average atomic mass of potassium, calculate the mass (in amu) of 8.70 × 106 potassium atoms. amu c. Using the average atomic mass of lithium, calculate the mass (in amu) of 3.40 × 1023 of lithium atoms. amu
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter5: Stoichiometry
Section: Chapter Questions
Problem 23Q: Reference Section 5-2 to find the atomic masses of 12C and 13C, the relative abundance of 12C and...
Related questions
Question
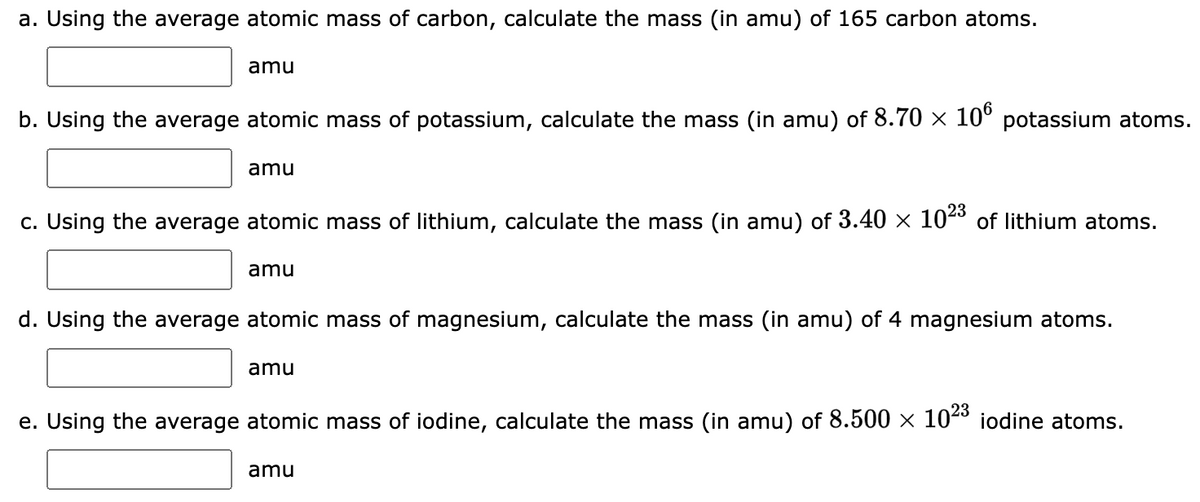
Transcribed Image Text:a. Using the average atomic mass of carbon, calculate the mass (in amu) of 165 carbon atoms.
amu
b. Using the average atomic mass of potassium, calculate the mass (in amu) of 8.70 × 106 potassium atoms.
amu
c. Using the average atomic mass of lithium, calculate the mass (in amu) of 3.40 × 10²³ of lithium atoms.
amu
d. Using the average atomic mass of magnesium, calculate the mass (in amu) of 4 magnesium atoms.
amu
e. Using the average atomic mass of iodine, calculate the mass (in amu) of 8.500 × 10²³ iodine atoms.
amu
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question

Transcribed Image Text:Using the average atomic mass of magnesium, calculate the mass (in amu) of 4 magnesium atoms.
amu
Using the average atomic mass of iodine, calculate the mass (in amu) of 8.500 × 1023 iodine atoms.
amu
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
