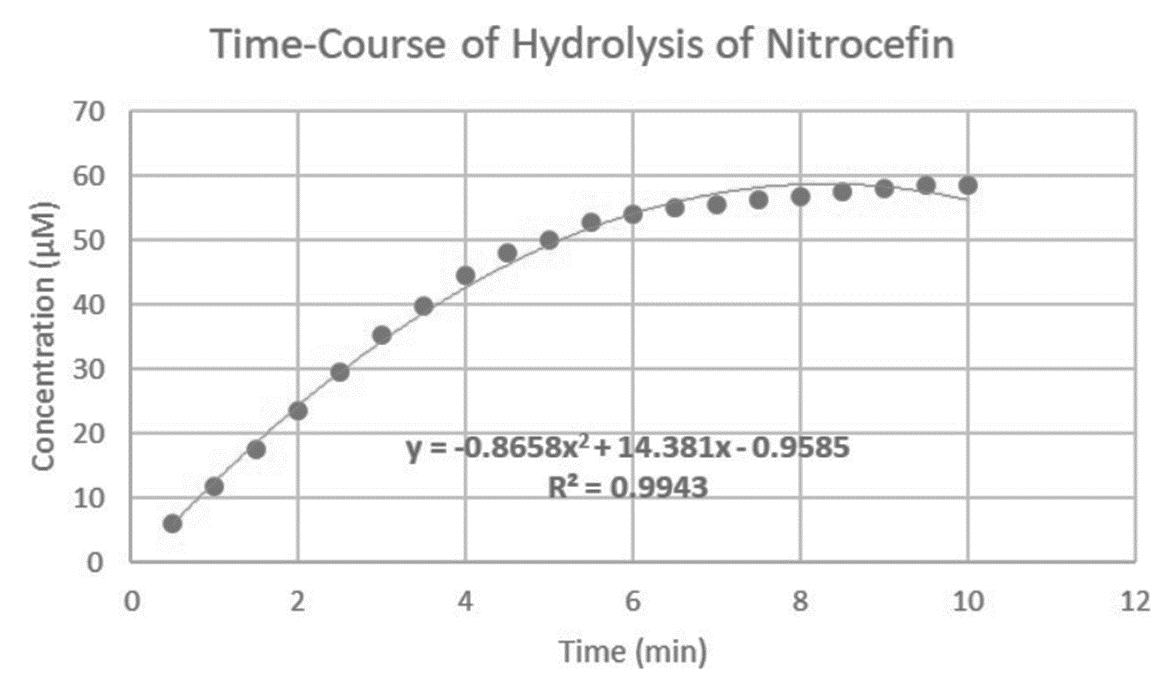
Using the data below (included for context), what is the equation for the trendline for the data points that fall in the linear range (linear range was found to be timepoints 1 min to 4 min,)? Your answer should be in the form of y = mx + b. Make sure that your trendline only includes the data points that fall in the linear range. In order to determine the trendline using Excel, you may need to create a new graph that only includes the earlier data points. With your new trendline, what is the initial velocity when 1 nM OXA-M290 is mixed with 60 μM nitrocefin? Make sure to include units in your answer.
Using the data below (included for context), what is the equation for the trendline for the data points that fall in the linear range (linear range was found to be timepoints 1 min to 4 min,)? Your answer should be in the form of y = mx + b. Make sure that your trendline only includes the data points that fall in the linear range. In order to determine the trendline using Excel, you may need to create a new graph that only includes the earlier data points. With your new trendline, what is the initial velocity when 1 nM OXA-M290 is mixed with 60 μM nitrocefin? Make sure to include units in your answer.
Context: DO NOT ANSWER
Concentration of hydrolyzed nitrocefin at 20 time points. Graph attached below
|
Time (min) |
Concentration (μM) |
|
0.5 |
6.01 |
|
1 |
11.78 |
|
1.5 |
17.6 |
|
2 |
23.51 |
|
2.5 |
29.58 |
|
3 |
35.31 |
|
3.5 |
39.73 |
|
4 |
44.5 |
|
4.5 |
48.18 |
|
5 |
50.05 |
|
5.5 |
52.72 |
|
6 |
54.01 |
|
6.5 |
55.06 |
|
7 |
55.65 |
|
7.5 |
56.39 |
|
8 |
56.74 |
|
8.5 |
57.49 |
|
9 |
58.03 |
|
9.5 |
58.61 |
|
10 |
58.69 |
Step by step
Solved in 4 steps with 7 images




