Using the data shown in the table, calculate the path radius of the Ca+ ion. 0.089 Ca+ path radius: m Mass Charge Isotope (x10-25 kg) (×10-19 C) Incorrect Ca+ 0.666 +1.602 Ca2+ 0.666 +3.204 Ba+ 2.28 +1.602 Ba2+ 2.28 +3.204 Ra+ 3.75 +1.602 Ra2+ 3.75 +3.204 Ra3+ 3.75 +4.806 Using the same data table, match the particles to their Path A Ca2+ Answer Bank path label. Path B Ca+ Path C Ba2+ Path D Ra2+ Path E Ra3+ Path F Ba+ Path G Ra+
Using the data shown in the table, calculate the path radius of the Ca+ ion. 0.089 Ca+ path radius: m Mass Charge Isotope (x10-25 kg) (×10-19 C) Incorrect Ca+ 0.666 +1.602 Ca2+ 0.666 +3.204 Ba+ 2.28 +1.602 Ba2+ 2.28 +3.204 Ra+ 3.75 +1.602 Ra2+ 3.75 +3.204 Ra3+ 3.75 +4.806 Using the same data table, match the particles to their Path A Ca2+ Answer Bank path label. Path B Ca+ Path C Ba2+ Path D Ra2+ Path E Ra3+ Path F Ba+ Path G Ra+
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter30: Nuclear Physics
Section: Chapter Questions
Problem 66P
Related questions
Question
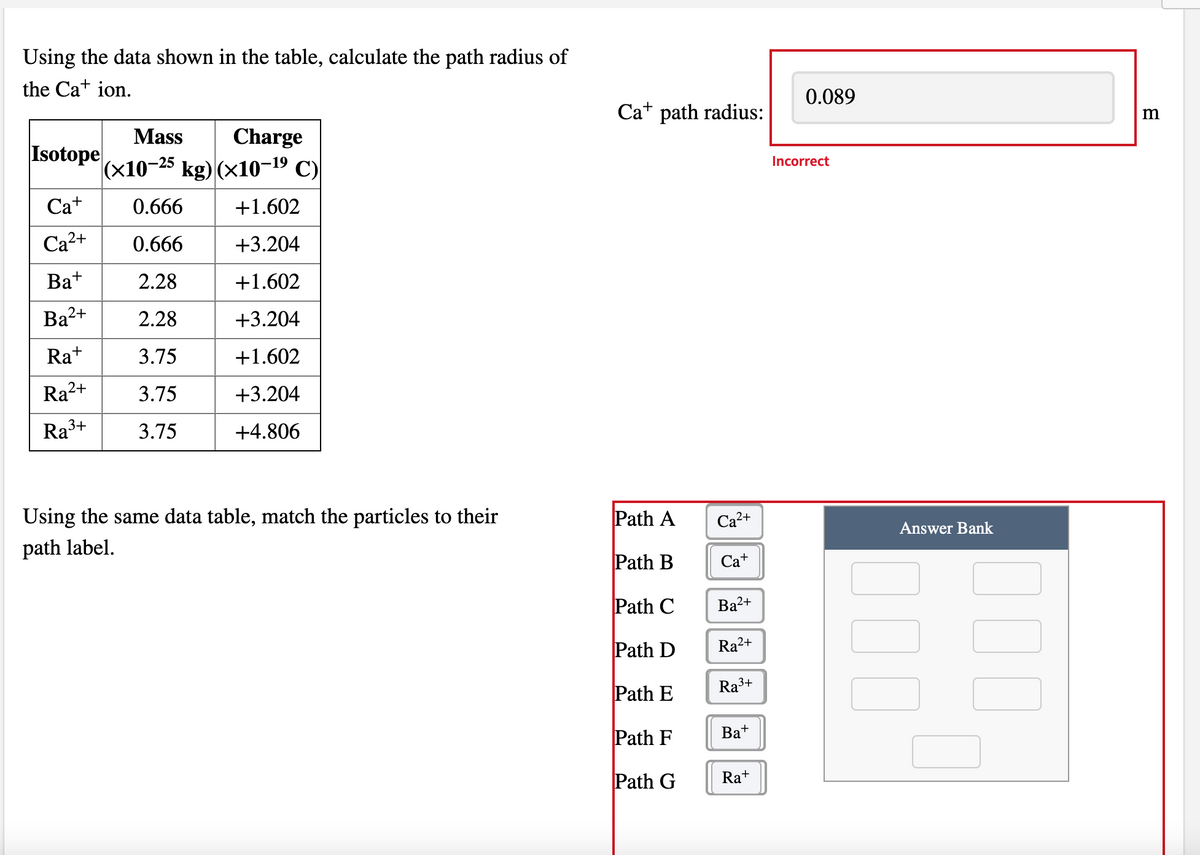
Transcribed Image Text:Using the data shown in the table, calculate the path radius of
the Ca+ ion.
0.089
Ca+ path radius:
Mass
Charge
m
Isotope
|(x10-25 kg) (x10–19 C)
Incorrect
Ca+
0.666
+1.602
Ca2+
0.666
+3.204
Ba+
2.28
+1.602
Ba2+
2.28
+3.204
Ra+
3.75
+1.602
Ra?
2+
3.75
+3.204
Ra3+
3.75
+4.806
Using the same data table, match the particles to their
Path A
Са2+
path label.
Answer Bank
Path B
Ca+
Path C
Ba2+
Path D
Ra²+
Path E
Ra3+
Path F
Bat
Path G
Ra+
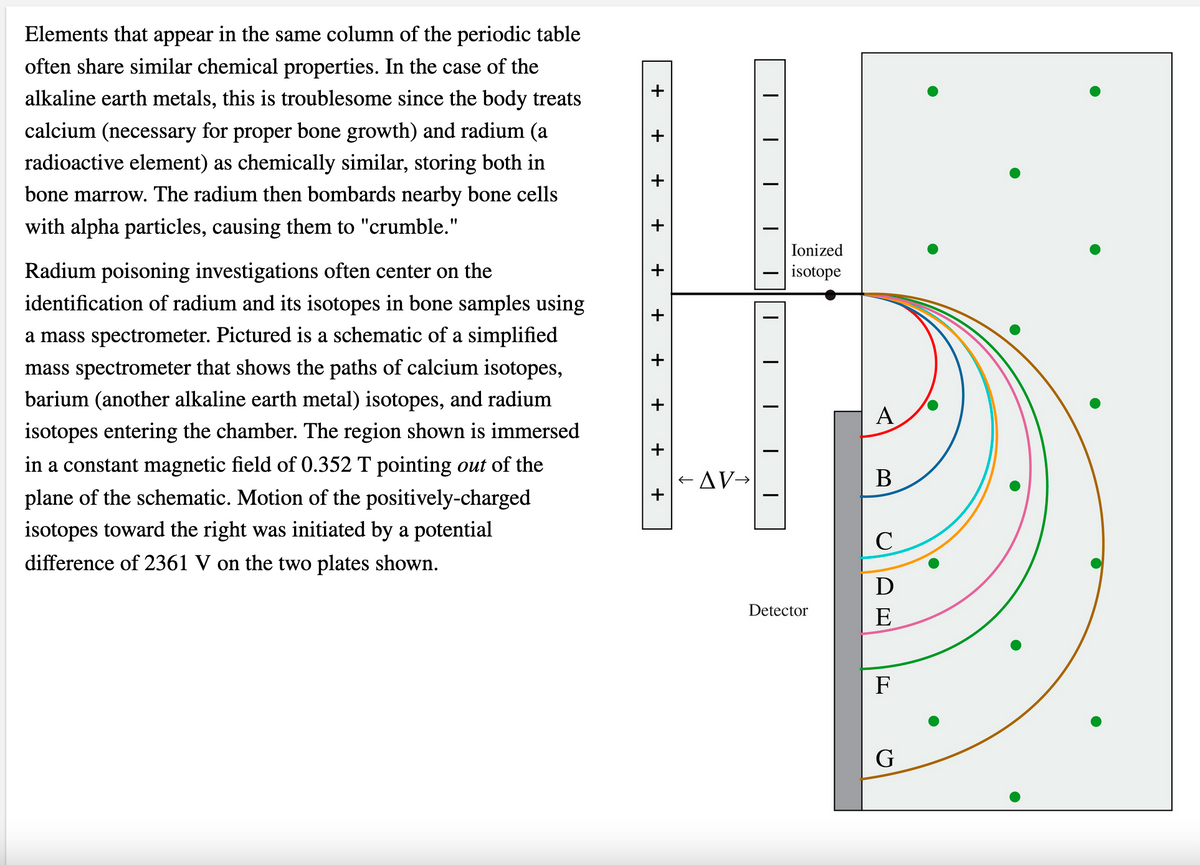
Transcribed Image Text:Elements that appear in the same column of the periodic table
often share similar chemical properties. In the case of the
alkaline earth metals, this is troublesome since the body treats
calcium (necessary for proper bone growth) and radium (a
radioactive element) as chemically similar, storing both in
bone marrow. The radium then bombards nearby bone cells
with alpha particles, causing them to "crumble."
Ionized
Radium poisoning investigations often center on the
identification of radium and its isotopes in bone samples using
isotope
a mass spectrometer. Pictured is a schematic of a simplified
mass spectrometer that shows the paths of calcium isotopes,
barium (another alkaline earth metal) isotopes, and radium
А
isotopes entering the chamber. The region shown is immersed
in a constant magnetic field of 0.352 T pointing out of the
+ AV→
В
plane of the schematic. Motion of the positively-charged
isotopes toward the right was initiated by a potential
difference of 2361 V on the two plates shown.
D
Detector
E
F
G
+
+
+
+
+
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning