Q: Please show mechanisms for following schemes
A: The mechanism of the following linear polymerization and the cross linked polymerization reactions o...
Q: 1. Balance the equation: - Cl: + KOH - KCI + KCIO, + H:O 2. The chemical name for Ag:CrO. 3. How man...
A: You have posted multipart of question as per the guidelines i have solved first three subparts kindl...
Q: Biomolecular Signaling Hydrogen sulfide and nitrogen monoxide can act as signaling agents in living ...
A: (a) Rate of reaction = change in conc of species / time taken For avg. conc.,lets take initial and ...
Q: A college chemistry student is solving this problem: "Determine the initial volume, in m³, of a gas ...
A:
Q: In a natural gas field, a cylindrical tank with a diameter of 5 meters and a height of 8 meters was ...
A: Given that , Radius of cylinder= r= 5 m Height of cylinder= h= 8 m Pressure= P= 1.7 atm Temperature=...
Q: Draw the organic product of the reaction. + OCH(CH32
A: Alkyl iodide reacts with alkoxide ions to form an ether.
Q: Mass of bromobenzene used (g) 4.567 Saved Amount of bromobenzene used (mol) 0.02909 Saved Mass of ma...
A:
Q: According to the rules that were discussed early this quarter, which molecule should be the stronger...
A:
Q: The following initial rate data are for the oxidation of arsenate ion by cerium(IV) ion in aqueous s...
A: For a reaction, A + B = C+ D The rate of the reaction will be, rate = k[A]x[B]y...
Q: According to the Arrhenius Theory the ion that is produced in the greatest amount when NaOH is disso...
A: 1) Correct option is (c) , when NaOH dissolved in water it gives OH- and Na+ 2)Correct option is (a)...
Q: Determine the pH and percent ionization of a .80 M HNO2 solution. Given Ka for HNO2 = 4.5*10^-4
A: pH is determined by the concentration of H+ ions that will be formed after ionization of Nitrous aci...
Q: Balance th
A:
Q: The pH of a 0.0057 M solution of Ca(OH)₂ is
A: Given- Concentration of Ca(OH)2= 0.0057 M
Q: When a weak acid such as acetic acid, HC2H3O2, is titrated with KOH what are ALL of the species in s...
A: The weak acids are the compounds that are weakly dissociated into water. However, strong acids or ba...
Q: Which of the following is the most stable alkene 1. 2 H,C HC H,C. HC. CH3 CH CH, A. two of these hav...
A: As the substituents (alkyl groups) on alkene incrases stability of alkene increases
Q: rite the formula of the conjugate base of the Brønsted-Lowry acid, H₂C₂O₄
A: H2C2O4
Q: A student was studying the pH differences in Erie Lake streams. The student measure a pH of 6 in str...
A:
Q: Question 20 of 25 View Policies Current Attempt in Progress Propose an efficient synthesis for the f...
A: This is a multi step synthesis reaction . Detail mechanistic pathway is given below by which we can ...
Q: For the decomposition of ammonia on a platinum surface at 856 °C 2 NH3(g) → N2(g) + 3 H2(g) the aver...
A: Given reaction : 2 NH3(g) → N2 (g) + 3 H2 (g) Average Rate of disappearance of NH3 = 1.50×10-6 M/s A...
Q: Why can a Pb sample accommodate more Sn atoms in its microstructure compared to how many atoms of Pb...
A: Lead (Pb) has an atomic number of 82 and a high density. Its crystal structure is face-centered cubi...
Q: The gas phase decomposition of sulfuryl chloride at 600 K so2Cl2(g) SO2(g) + Cl2(g) is first order i...
A:
Q: Determine the formula and name for a compound that is 42.9% carbon and 57.1% Oxygen.
A:
Q: Consider the initial conditions of a gas: Initial Variable Value 400. K 250. V 3.30 atm Determine th...
A:
Q: what is the polymer formed when resorcinol and formaldehyde are combined with naoh? Shows complete ...
A: Please find the below attachment.
Q: The Mole Concept Sulfur compounds are converted to H2SO4 while NO, compounds are converted to HNO3, ...
A: Given reaction is : NH4+ + 2O2 --------> NO3- + H2O + 2H+
Q: pH Versus Hydronium lon Concentration 14 12 Bleach. Aqueous ammonia • 10 Milk of magnesia • 8- Seawa...
A: pH vs Hydronium ion concentration is given for some solutions and natural substances.
Q: QUESTION 3 For the following expressions, how many significant figures will the answers have: a) (0....
A:
Q: The ideal gas law would work for which if the following? a. A fluid with Z = 0.7 b. Hydrogen...
A: An ideal gas follow the ideal gas law, which is given by PV=nRT, where P, V, and n are the pressure,...
Q: QUESTION 2 Typically, electrochemical measurements are expressed relative to the Standard Hydrogen E...
A: Answer: These questions are based on the basic understanding of electrochemical cell. Since it is no...
Q: QUESTION 9 According to the following reaction, how many moles of sodium bromide should be left when...
A:
Q: volume
A:
Q: Which of the following aqueous solutions are good buffer systems? 0.30 M ammonia + 0.34 M potassium ...
A:
Q: Q2. A. Compound Which is composed of 57.14 % C, 6.16% H, 9.52 % N, 27.18% O by mass and has a molar ...
A: Given, Mass percentage of C = 57.14% Mass percentage of H = 6.16% Mass percentage of N = 9.52% M...
Q: A 4.0 MG/dL standard solution is needed. To prepare is 100ML of the working standards, how much stoc...
A: Given, A 4.0 mg/dL standard solution is needed. To prepare is 100 mL of the working standards, the ...
Q: Compounds A and B react to form Cand D in a reaction that is found to be second-order overall and se...
A:
Q: 1. Balance the equation: - Cl; + KOH KCI + KCIO, + H;o 2. The chemical name for Ag:CrOs 3. How many ...
A:
Q: A buffer system with pKa of 6.4. Which of the following pH is reasonable when a base is added to the...
A: Buffer solution: The solution that resists the change in pH is known as a buffer solution. The buffe...
Q: 1. Balance the equation: - Cl; + KOH - KCI + KCIO, + H.O 2. The chemical name for Ag:CrO. 3. How man...
A:
Q: a) Determine the grams of reagent in excess if 4.19 moles BiCl3 reacted with 0.970 moles water. b) W...
A:
Q: 1. Balance the equation: - C2H2 + O2 → CO2 + H2O 2. The chemical name for AAL2(SO4) 3. How many prot...
A: Since you have posted multiple questions, the answer for first three question is given below. Kindly...
Q: 10. Choose two of the following electron configurations to identify the ion or atom. Be sure to expl...
A:
Q: AG° = %3D
A:
Q: Which ether cannot be prepared via a Williamson ether synthesis? OA di-1-naphthyl ether OR 15-crown-...
A: Williamson ether synthesis is an SN2 reaction between an organohalide and an alkoxide to form a ethe...
Q: A solid substance has a mass of 150 grams. It is cooled from 25°C to 5°C and loses 8.675 kJ of heat....
A:
Q: onsider the chemical reaction where hydrochloric acid (HCl) reacts with the antacid magnesium hydrox...
A: Given, Reaction between hydrochloric acid (HCl) and antacid magnesium hydroxide .
Q: Professor Rowan is preparing for his experiment in Sinnoh Lab. The structures of the substances that...
A: London-dispersion forces are present in all the molecules. Dipole-dipole forces are present in molec...
Q: Question 4 Status: Not yet answered | Points possible: 1.00 Burning 1.02 g of a fuel causes the wate...
A: We know that Heat released = C*ΔT (because here calorimeter is used ) Given - - > C = 3.09 KJ/°...
Q: irection (forward or reverse) that the reaction will go in order to re-establish 3 gas is added to t...
A: Effect of change on equilibrium is determined by Le-Chateleir principle. The law states that the equ...
Q: Example: A solution buffered at pH 3.90 is needed for a reaction. Would formic acid and its salt, so...
A: Given: A solution buffered at pH 3.90 is required for a reaction. ka of formic acid is 1.8 x 10-4
Q: Graph 1 Graph 2 Graph 3 Timo Timo Timo
A:
Using the
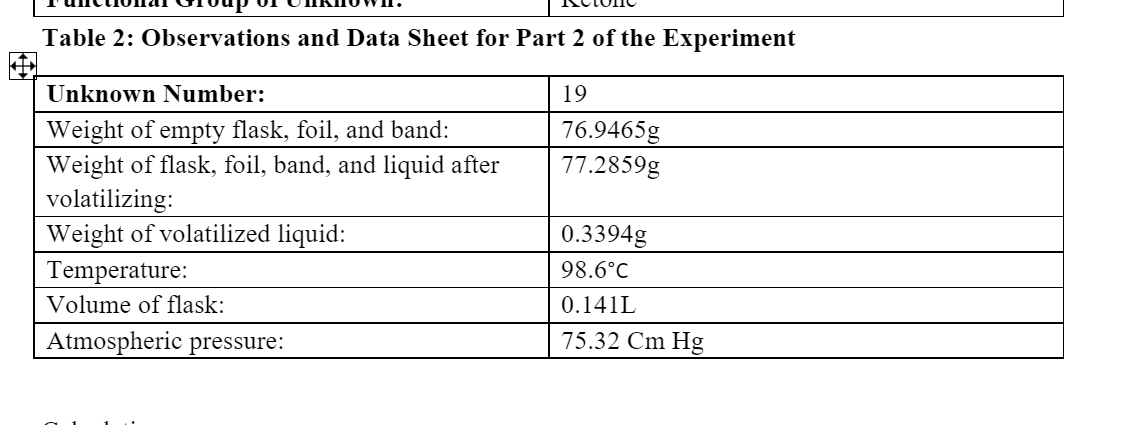
Step by step
Solved in 2 steps

- Derive the equations for the critical parameters VC, TC, and PC, (in terms of a, b and R) for the Berthelot EOS and then calculate the critical compression factor, ZC for the Berthelot EOS.For each of the following statements, answer TRUE or FALSE and explain your answer briefly. Corrections for water density, thermal expansion, and buoyancy difference are always required for the calibration of volumetric flask.A fly ash aerosol of monodisperse particles with a density of 2.0 g/mL and particle diameter of 10.0 mm has a concentration of 1000.0 mg/m3. Calculate the settling velocity in cm/s and the settling rate in g/m2-s. Please answer very soon will give rating surely
- A student doing this experiment had some problems with the procedure, as described below. Explain what effect each problem would have had on the magnitude of the results calculated. a) The vial containing the Mg tipped over when the student first placed it in the Erlenmeyer flask. The student was able to return it to an upright position almost immediately, and the student continued the experiment. b) The student neglected to open the pinch clamp on the plastic tubing between the filter flask and the beaker when the Mg came in contact with the HCl solution. After the stopper popped out of the Erlenmeyer flask, the student quickly replaced the stopper, opened the clamp, and proceeded with the experiment. c) The student began titrating the unreacted HCl with standard NaOH solution and realized that there was a large air bubble in the buret tip. The bubble came out as the first few milliliters of titrant were added. d) The student forgot to add indicator to the titration mixture until…please help this is my 4th time posting this and i havent gotten a correct answer. Please actually draw the graph out. A newly discovered enzyme, MBCase, cleaves carbon-carbon bonds of vicinal alcohol; The kinetic parameters were measured for 1.0 nM Of MBGase and determined to be a Km = 10 nM and a kcat = 100 s-1 a.) Sketch the graph out as a double-reciprocal plot (Lineweaver-Burke Plot) and label: -the axes (with units) -the point on the curve that can be used to determine Km -the point on the curve that can be used to determine VmaxIndicate the order of elution of the following compounds from a normal-phase packed HPLC column a. ethyl acetate, acetic acid, dimethylamineb. propylene, hexane, benzene, dichlorobenzene
- It is emphasized in the procedure that the water bath should be continuously stirred while heating and cooling the sample. What will happen if the water bath was not stirred? Identify its effect to the related derived dataCalculate the values of vrms, vmp, and vmean for Cl2 (70.90 g mol-1) at 25 °CA typical split ratio is in ........ratio for .........quantity of sample?

