Using the standard thermodynamic values in the Appendix, calculate the theoretical values of AH°, AS°, and AG for the reaction of Ca(OH)2 dissolving in water. Discuss the sign of each and what it tells you about this reaction.
Using the standard thermodynamic values in the Appendix, calculate the theoretical values of AH°, AS°, and AG for the reaction of Ca(OH)2 dissolving in water. Discuss the sign of each and what it tells you about this reaction.
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.28QAP
Related questions
Question
#5 plz
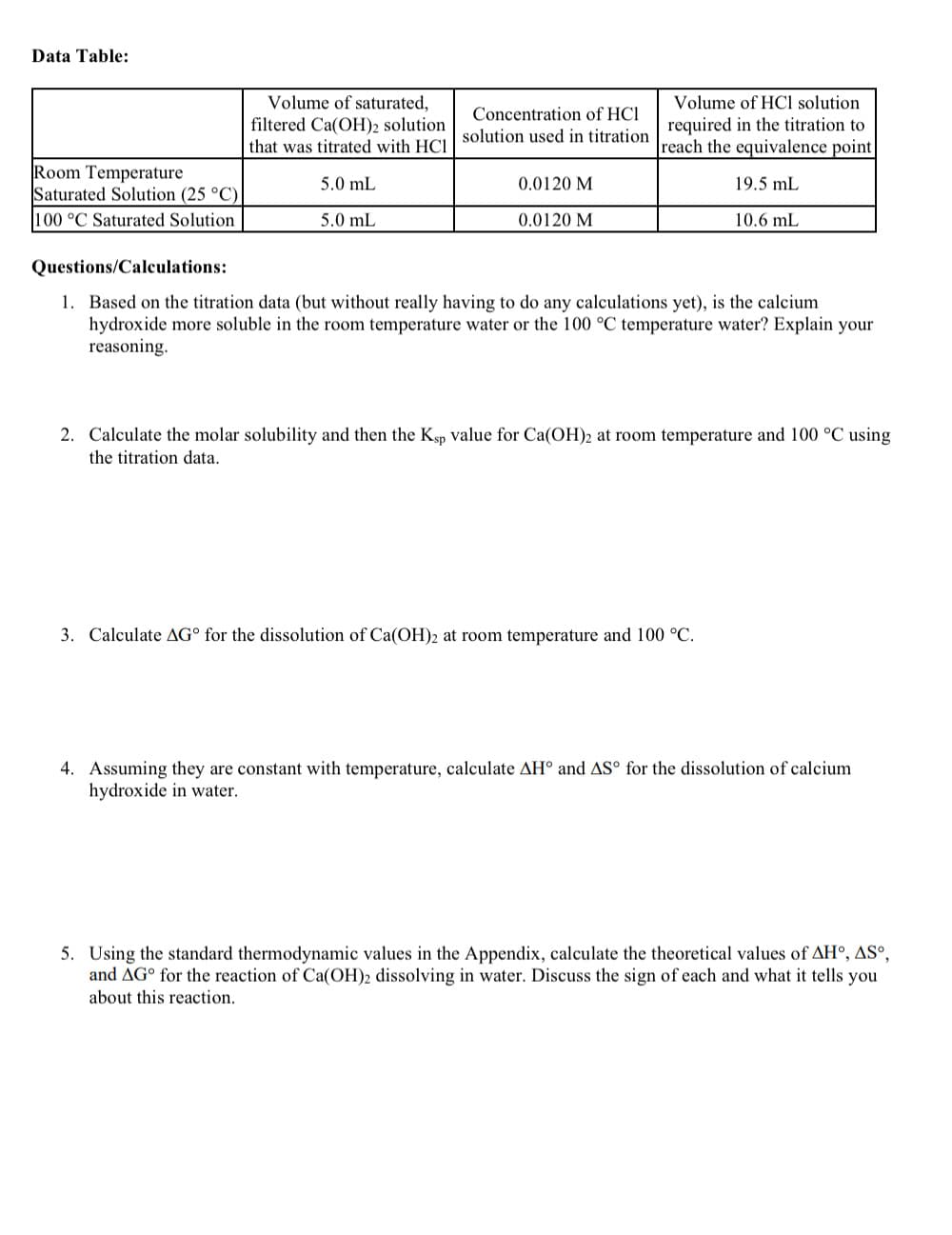
Transcribed Image Text:Data Table:
Room Temperature
Saturated Solution (25 °C)
100 °C Saturated Solution
Volume of saturated,
filtered Ca(OH)2 solution
that was titrated with HCI
5.0 mL
5.0 mL
Volume of HCl solution
Concentration of HCI
solution used in titration required in the titration to
reach the equivalence point
19.5 mL
10.6 mL
0.0120 M
0.0120 M
Questions/Calculations:
1. Based on the titration data (but without really having to do any calculations yet), is the calcium
hydroxide more soluble in the room temperature water or the 100 °C temperature water? Explain your
reasoning.
2. Calculate the molar solubility and then the Ksp value for Ca(OH)2 at room temperature and 100 °C using
the titration data.
3. Calculate AG for the dissolution of Ca(OH)2 at room temperature and 100 °C.
4. Assuming they are constant with temperature, calculate AH° and AS° for the dissolution of calcium
hydroxide in water.
5. Using the standard thermodynamic values in the Appendix, calculate the theoretical values of AH°, AS°,
and AG for the reaction of Ca(OH)2 dissolving in water. Discuss the sign of each and what it tells you
about this reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning



Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning