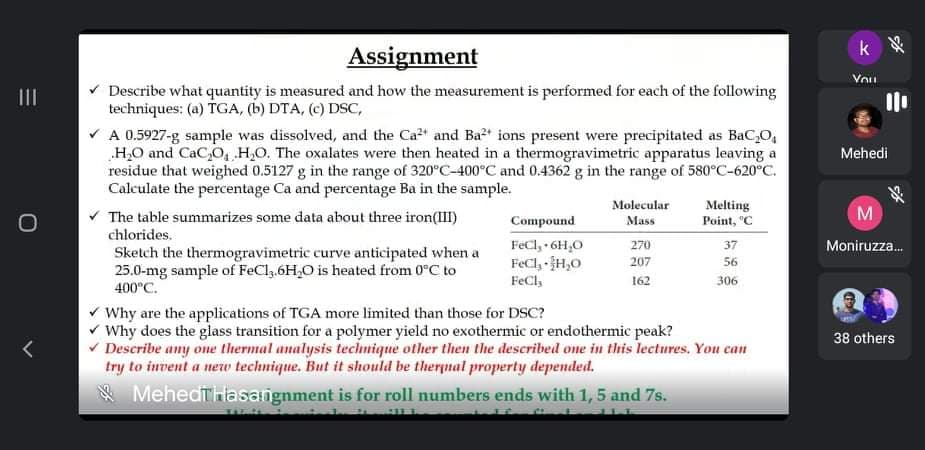
v Describe what quantity is measured and how the measurement is performed for each of the following techniques: (a) TGA, (b) DTA, (c) DSC,
v Describe what quantity is measured and how the measurement is performed for each of the following techniques: (a) TGA, (b) DTA, (c) DSC,
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Transcribed Image Text:k
Assignment
Vou
* Describe what quantity is measured and how the measurement is performed for each of the following
techniques: (a) TGA, (b) DTA, (c) DSC,
V A 0.5927-g sample was dissolved, and the Ca2" and Ba2t ions present were precipitated as BaC,O,
„H,O and CaC,O, H,O. The oxalates were then heated in a thermogravimetric apparatus leaving a
residue that weighed 0.5127 g in the range of 320°C-400°C and 0.4362 g in the range of 580°C-620°C.
Calculate the percentage Ca and percentage Ba in the sample.
v The table summarizes some data about three iron(III)
II
Mehedi
Molecular
Melting
Point, "C
M
Compound
Mass
chlorides.
FeCl, 6H,0
FeCl, H,0
FeCl,
270
37
Moniruzza.
Sketch the thermogravimetric curve anticipated when a
25.0-mg sample of FeCl,.6H,0 is heated from 0°C to
400°C.
207
56
162
306
V Why are the applications of TGA more limited than those for DSC?
v Why does the glass transition for a polymer yield no exothermic or endothermic peak?
v Describe any one thermal analysis technique other then the described ome in this lectures. You can
try to invent a new technique. But it should be therpnal property depended.
* Mehedthlasangnment is for roll numbers ends with 1, 5 and 7s.
38 others
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The