V Figure 4.5 Four ways that carbon skeletons can vary. (a) Length H H HH H H- -H Ethane Propane Carbon skeletons vary In length. ы вranching H -- HHHH H H HAA H HA 2-Methylpropane (commonly called isobutane) Butane Skeletons may be unbranched or branched. () Double bond position HHHH 1-Butene 2-Butene The skeleton may have double bonds, which can vary in location. (d) Presence of rings H. H" H- Cycdohexane Benzene Some carbon skeletons are arranged in rings. In the abbreviated structural formula for each compound to its right), each comer represents a carbon and its attached hydrogers. V Figure 4.7 Three types of isomers. Isomers are compounds that have the same molecular formula but different structures. (a) Structural Isomers H-CH HH H H H H H H HH H H H H H H H 2-Methylbutane Structural isomers differ in covalent partners, as shown In ths Pentane example of two isomers of C,H2. (b) Cis-trans Isomers H C=C H cis isomer. The two Xs are on trans Isomer: The two Xs are the same side. on opposite sides. Cs-trans isomers differ in arrangement about a double bond. In these diagrams, X represents an atom or group of atoms attached to a double bonded carbon.
V Figure 4.5 Four ways that carbon skeletons can vary. (a) Length H H HH H H- -H Ethane Propane Carbon skeletons vary In length. ы вranching H -- HHHH H H HAA H HA 2-Methylpropane (commonly called isobutane) Butane Skeletons may be unbranched or branched. () Double bond position HHHH 1-Butene 2-Butene The skeleton may have double bonds, which can vary in location. (d) Presence of rings H. H" H- Cycdohexane Benzene Some carbon skeletons are arranged in rings. In the abbreviated structural formula for each compound to its right), each comer represents a carbon and its attached hydrogers. V Figure 4.7 Three types of isomers. Isomers are compounds that have the same molecular formula but different structures. (a) Structural Isomers H-CH HH H H H H H H HH H H H H H H H 2-Methylbutane Structural isomers differ in covalent partners, as shown In ths Pentane example of two isomers of C,H2. (b) Cis-trans Isomers H C=C H cis isomer. The two Xs are on trans Isomer: The two Xs are the same side. on opposite sides. Cs-trans isomers differ in arrangement about a double bond. In these diagrams, X represents an atom or group of atoms attached to a double bonded carbon.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter2: Chemical Compounds
Section2.9: Organic Molecular Compounds
Problem 2.12E
Related questions
Question
See Figures 4.5a and 4.7. Can propane (C3H8) form isomers? Explain.
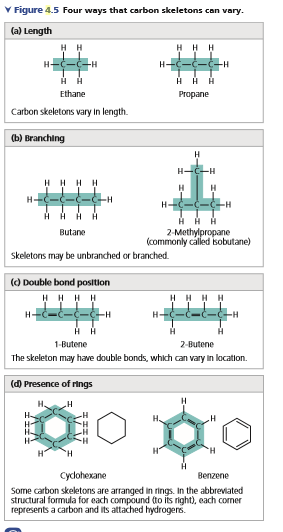
Transcribed Image Text:V Figure 4.5 Four ways that carbon skeletons can vary.
(a) Length
H H
HH H
H-
-H
Ethane
Propane
Carbon skeletons vary In length.
ы вranching
H
--
HHHH
H
H
HAA
H HA
2-Methylpropane
(commonly called isobutane)
Butane
Skeletons may be unbranched or branched.
() Double bond position
HHHH
1-Butene
2-Butene
The skeleton may have double bonds, which can vary in location.
(d) Presence of rings
H.
H"
H-
Cycdohexane
Benzene
Some carbon skeletons are arranged in rings. In the abbreviated
structural formula for each compound to its right), each comer
represents a carbon and its attached hydrogers.
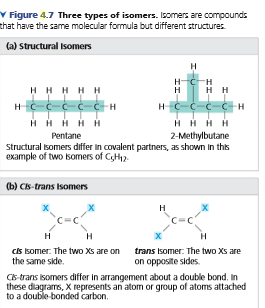
Transcribed Image Text:V Figure 4.7 Three types of isomers. Isomers are compounds
that have the same molecular formula but different structures.
(a) Structural Isomers
H-CH
HH H H H
H H
H
HH H H H
H H H H
2-Methylbutane
Structural isomers differ in covalent partners, as shown In ths
Pentane
example of two isomers of C,H2.
(b) Cis-trans Isomers
H
C=C
H
cis isomer. The two Xs are on trans Isomer: The two Xs are
the same side.
on opposite sides.
Cs-trans isomers differ in arrangement about a double bond. In
these diagrams, X represents an atom or group of atoms attached
to a double bonded carbon.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning