► View Available Hint(s) 1 mol of oxygen gas at 273 K and 40 L Greatest entropy 1/2 mol of neon gas at 100 K and 20 L The correct ranking cannot be determined. 1 mol of neon gas at 273 K and 40 L 1/2 mol of neon gas at 273 K and 20 L 1/2 mol of liquid neon at 100 K 1 mol of carbon disulfide gas at 273 K and 40 L Reset 1 mol of neon gas at 273 K and 20 L Help Least entropy
► View Available Hint(s) 1 mol of oxygen gas at 273 K and 40 L Greatest entropy 1/2 mol of neon gas at 100 K and 20 L The correct ranking cannot be determined. 1 mol of neon gas at 273 K and 40 L 1/2 mol of neon gas at 273 K and 20 L 1/2 mol of liquid neon at 100 K 1 mol of carbon disulfide gas at 273 K and 40 L Reset 1 mol of neon gas at 273 K and 20 L Help Least entropy
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter10: Entropy And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 10.33PAE: According to Lambert, leaves lying in the yard and playing cards that are in disarray on a table...
Related questions
Question
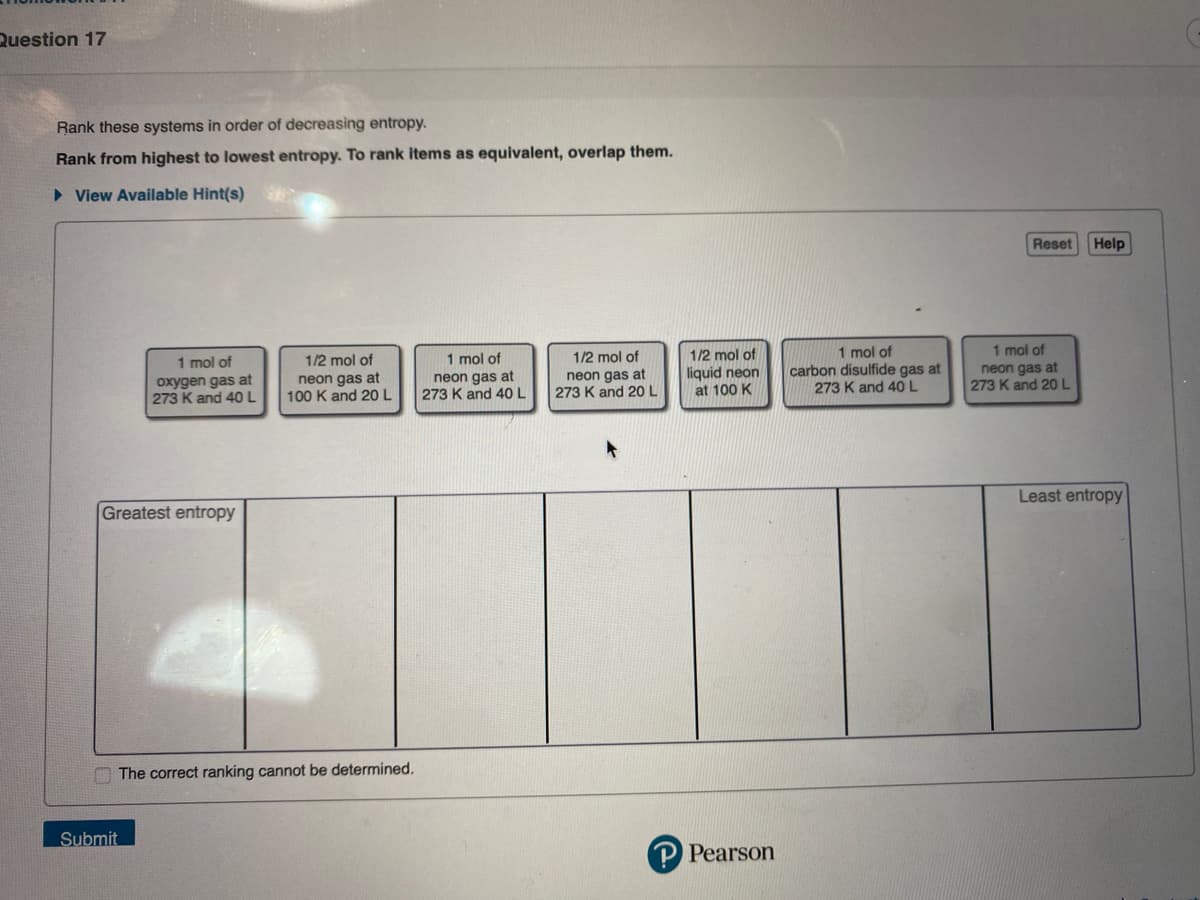
Transcribed Image Text:Question 17
Rank these systems in order of decreasing entropy.
Rank from highest to lowest entropy. To rank items as equivalent, overlap them.
► View Available Hint(s)
1 mol of
oxygen gas at
273 K and 40 L
Greatest entropy
Submit
1/2 mol of
neon gas at
100 K and 20 L
The correct ranking cannot be determined.
1 mol of
neon gas at
273 K and 40 L
1/2 mol of
neon gas at
273 K and 20 L
1/2 mol of
liquid neon
at 100 K
P Pearson
1 mol of
carbon disulfide gas at
273 K and 40 L
Reset
1 mol of
neon gas at
273 K and 20 L
Help
Least entropy
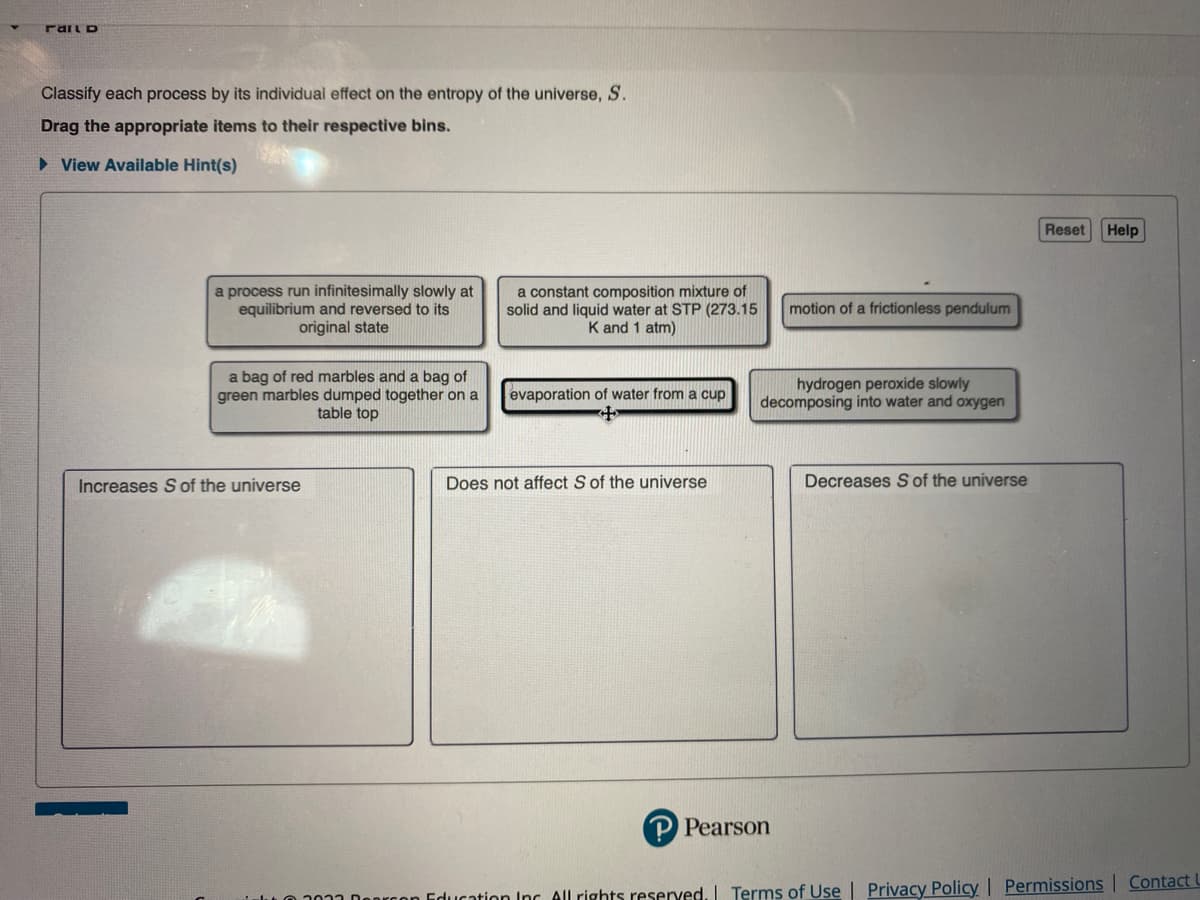
Transcribed Image Text:Mail D
Classify each process by its individual effect on the entropy of the universe, S.
Drag the appropriate items to their respective bins.
►View Available Hint(s)
a process run infinitesimally slowly at
equilibrium and reversed to its
original state
a bag of red marbles and a bag of
green marbles dumped together on a
table top
Increases S of the universe
a constant composition mixture of
solid and liquid water at STP (273.15
K and 1 atm)
evaporation of water from a cup
+
Does not affect S of the universe
motion of a frictionless pendulum
hydrogen peroxide slowly
decomposing into water and oxygen
P Pearson
Decreases S of the universe
Reset
Help
con Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions Contact L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning