Waste acid of pH 3.0 is stored in a lead-lined vessel, as shown in the figure below. If the walls of the container undergo corrosion by the following reaction: Pb(s) + 2H*(aq) → Pb³"(aq) + H;(g) what is the equilibrium concentration of dissolved Pb at 25°C if the pressure of hydrogen gas in the closed vessel is 0.5 atm? The AG; for Pb (aq) is -5,810 cal/mol. P) -0s am Waste acid p-30 Lead-lined tuk
Waste acid of pH 3.0 is stored in a lead-lined vessel, as shown in the figure below. If the walls of the container undergo corrosion by the following reaction: Pb(s) + 2H*(aq) → Pb³"(aq) + H;(g) what is the equilibrium concentration of dissolved Pb at 25°C if the pressure of hydrogen gas in the closed vessel is 0.5 atm? The AG; for Pb (aq) is -5,810 cal/mol. P) -0s am Waste acid p-30 Lead-lined tuk
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section17.6: Equilibria Involving Complex Ions
Problem 2.1ACP: Phosphate ions are abundant in cells, both as the ions themselves and as important substituents on...
Related questions
Question
100%
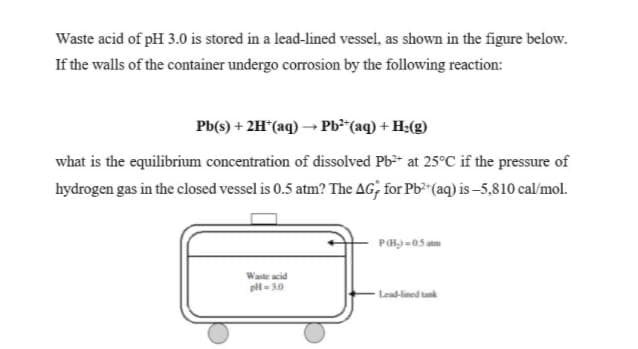
Transcribed Image Text:Waste acid of pH 3.0 is stored in a lead-lined vessel, as shown in the figure below.
If the walls of the container undergo corrosion by the following reaction:
Pb(s) + 2H*(aq) → Pb*"(aq) + H;(g)
what is the equilibrium concentration of dissolved Pb-* at 25°C if the pressure of
hydrogen gas in the closed vessel is 0.5 atm? The AG; for Pb (aq) is –5,810 cal/mol.
Waste acid
pl- 30
Lead-lined tunk
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning