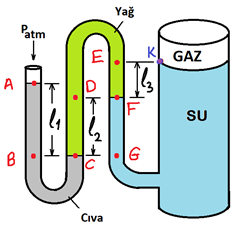
Water pressurized with a gas in a cylindrical tank are available. It is measured as l1 = 60 cm, l2 = 40 cm and l3 = 30 cm. Atmospheric pressure: Patm = 100 kPa ; Relative densities are: Dsu = 1, Poil = 0.85 and Pluc = 13.6. What is the difference between the pressure of the gas in the tank and the pressure at point A? a) 47.09 kPa B) -26.23 kPa NS) -6.20 kPa D) -52.81 kPa TO) 73.77 kPa
Water pressurized with a gas in a cylindrical tank are available. It is measured as l1 = 60 cm, l2 = 40 cm and l3 = 30 cm. Atmospheric pressure: Patm = 100 kPa ; Relative densities are: Dsu = 1, Poil = 0.85 and Pluc = 13.6. What is the difference between the pressure of the gas in the tank and the pressure at point A? a) 47.09 kPa B) -26.23 kPa NS) -6.20 kPa D) -52.81 kPa TO) 73.77 kPa
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Kreith, Frank; Manglik, Raj M.
Chapter4: Numerical Analysis Of Heat Conduction
Section: Chapter Questions
Problem 4.25P
Related questions
Topic Video
Question
Water pressurized with a gas in a cylindrical tank
are available. It is measured as l1 = 60 cm, l2 = 40 cm and l3 = 30 cm.
Atmospheric pressure: Patm = 100 kPa ; Relative densities are: Dsu = 1, Poil = 0.85 and Pluc = 13.6. What is the difference between the pressure of the gas in the tank and the pressure at point A?
|
||
|
||
|
||
|
||
|
22
Transcribed Image Text:Yağ
Patm
K
GAZ
A
F
SU
G
Civa
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning

Principles of Heat Transfer (Activate Learning wi…
Mechanical Engineering
ISBN:
9781305387102
Author:
Kreith, Frank; Manglik, Raj M.
Publisher:
Cengage Learning