Wavelength (m) · 10-11 10-9 10-7 10-5 10-3 10-1 101 103 Gamma! rays TUltra- i violet X rays Infrared iMicrowavesi Radio frequency 1020 1018 1016 1014 1012 1010 108 106 104 Frequency (s~1) Visible region 400 500 600 A Figure 6.4 The electromagnetic spectrum.* Wavelengths in the spectrum range from very short gamma rays to very long radio waves. 700 750 nm Visible
Wavelength (m) · 10-11 10-9 10-7 10-5 10-3 10-1 101 103 Gamma! rays TUltra- i violet X rays Infrared iMicrowavesi Radio frequency 1020 1018 1016 1014 1012 1010 108 106 104 Frequency (s~1) Visible region 400 500 600 A Figure 6.4 The electromagnetic spectrum.* Wavelengths in the spectrum range from very short gamma rays to very long radio waves. 700 750 nm Visible
Chapter7: Light And Color
Section: Chapter Questions
Problem 18E: Classify each of the following lasers as to type (solid-state, gas, dye, or semiconductor), and list...
Related questions
Question
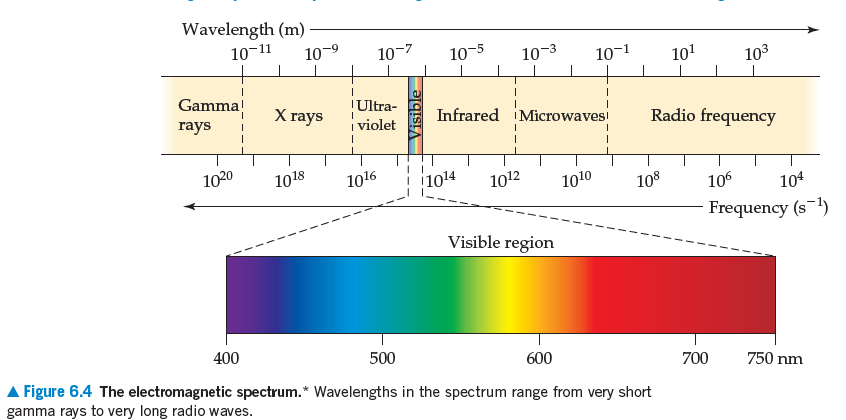
A popular kitchen appliance produces
with a frequency of 2450 MHz. With reference to Figure
6.4, answer the following: (a) Estimate the wavelength
of this radiation. (b) Would the radiation produced by the
appliance be visible to the human eye? (c) If the radiation
is not visible, do photons of this radiation have more or less
energy than photons of visible light? (d) Which of the following
is the appliance likely to be? (i) A toaster oven, (ii) A
microwave oven, or (iii) An electric hotplate.
Transcribed Image Text:Wavelength (m) ·
10-11
10-9
10-7
10-5
10-3
10-1
101
103
Gamma!
rays
TUltra-
i violet
X rays
Infrared iMicrowavesi
Radio frequency
1020
1018
1016
1014
1012
1010
108
106
104
Frequency (s~1)
Visible region
400
500
600
A Figure 6.4 The electromagnetic spectrum.* Wavelengths in the spectrum range from very short
gamma rays to very long radio waves.
700
750 nm
Visible
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning