We could also write this reaction three other ways, listed below. The equilibrium constants for all of the reactions are related. Write the equilibrium constant for each new reaction in terms of K, the equilibrium constant for the reaction above.
We could also write this reaction three other ways, listed below. The equilibrium constants for all of the reactions are related. Write the equilibrium constant for each new reaction in terms of K, the equilibrium constant for the reaction above.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter21: Chemistry Of The Main-group Elements
Section: Chapter Questions
Problem 21.200QP
Related questions
Question
Transcribed Image Text:m/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take
[Review Topics)
[References)
Use the References to access important values if needed for this question.
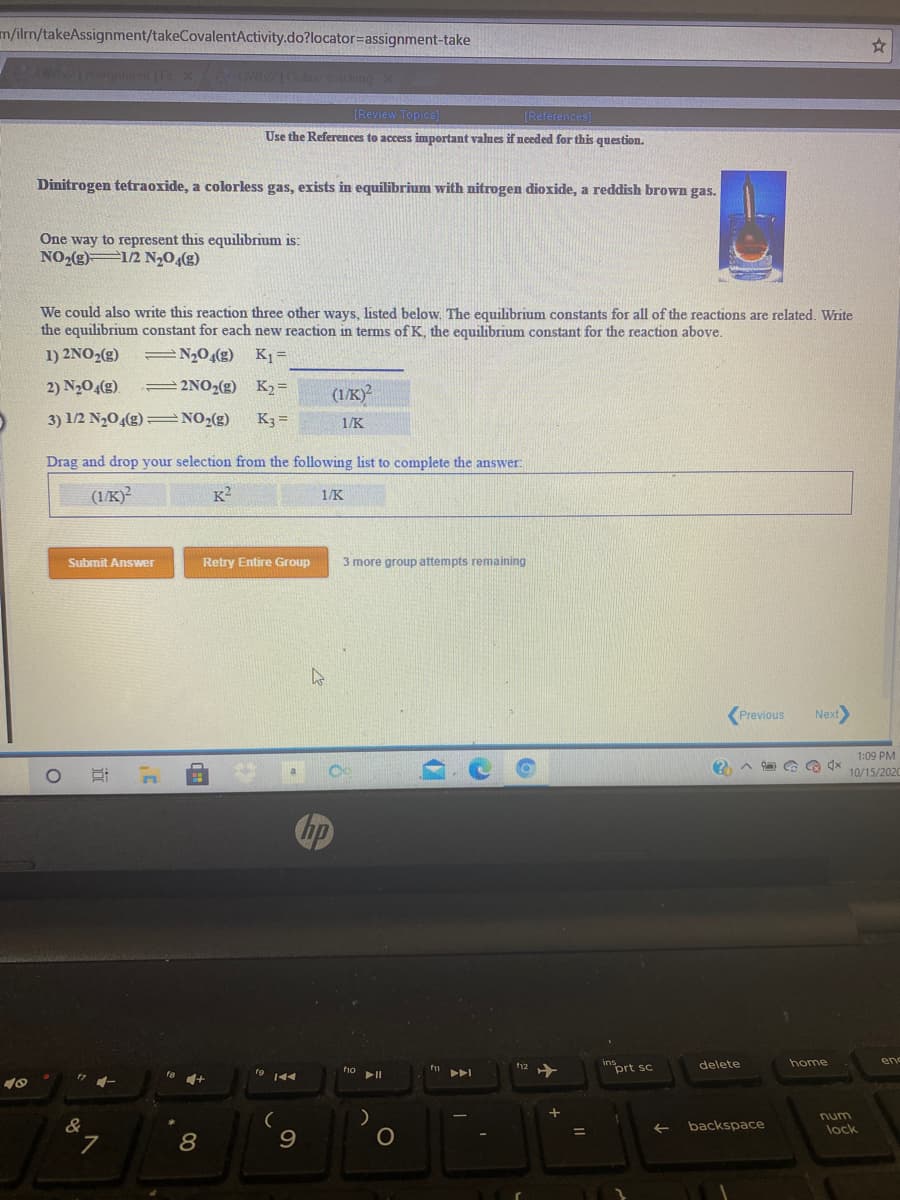
Dinitrogen tetraoxide, a colorless gas, exists in equilibrium with nitrogen dioxide, a reddish brown gas.
One way to represent this equilibrium is:
NO2(g) 1/2 N20,(g)
We could also write this reaction three other ways, listed below. The equilibrium constants for all of the reactions are related. Write
the equilibrium constant for each new reaction in terms of K, the equilibrium constant for the reaction above.
1) 2NO2(g)
N20(g)
K1 =
2) N204(g).
=2NO2(g) K2=
(1/K)
3) 1/2 N204(g) = NO2(g)
K3 =
1/K
Drag and drop your selection from the
owing list to complete the answer:
(1/K)?
K2
1/K
Submit Answer
Retry Entire Group
3 more group attempts remaining
Previous
Next
1:09 PM
? A G O 4
10/15/2020
prt sc
delete
home
enc
f12
19 11
40
num
backspace
lock
8
9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning