What amount of heat, in kJ, is required to convert 4.00 g of water at 67.0 °C to 4.00 g of steam at 100.0 °C? (specific heat capacity of water = 4.184 J/g °C; AHvap = 40.7 kJ/mol) %3D
What amount of heat, in kJ, is required to convert 4.00 g of water at 67.0 °C to 4.00 g of steam at 100.0 °C? (specific heat capacity of water = 4.184 J/g °C; AHvap = 40.7 kJ/mol) %3D
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter11: Liquids And Solids
Section: Chapter Questions
Problem 11.99QE
Related questions
Question
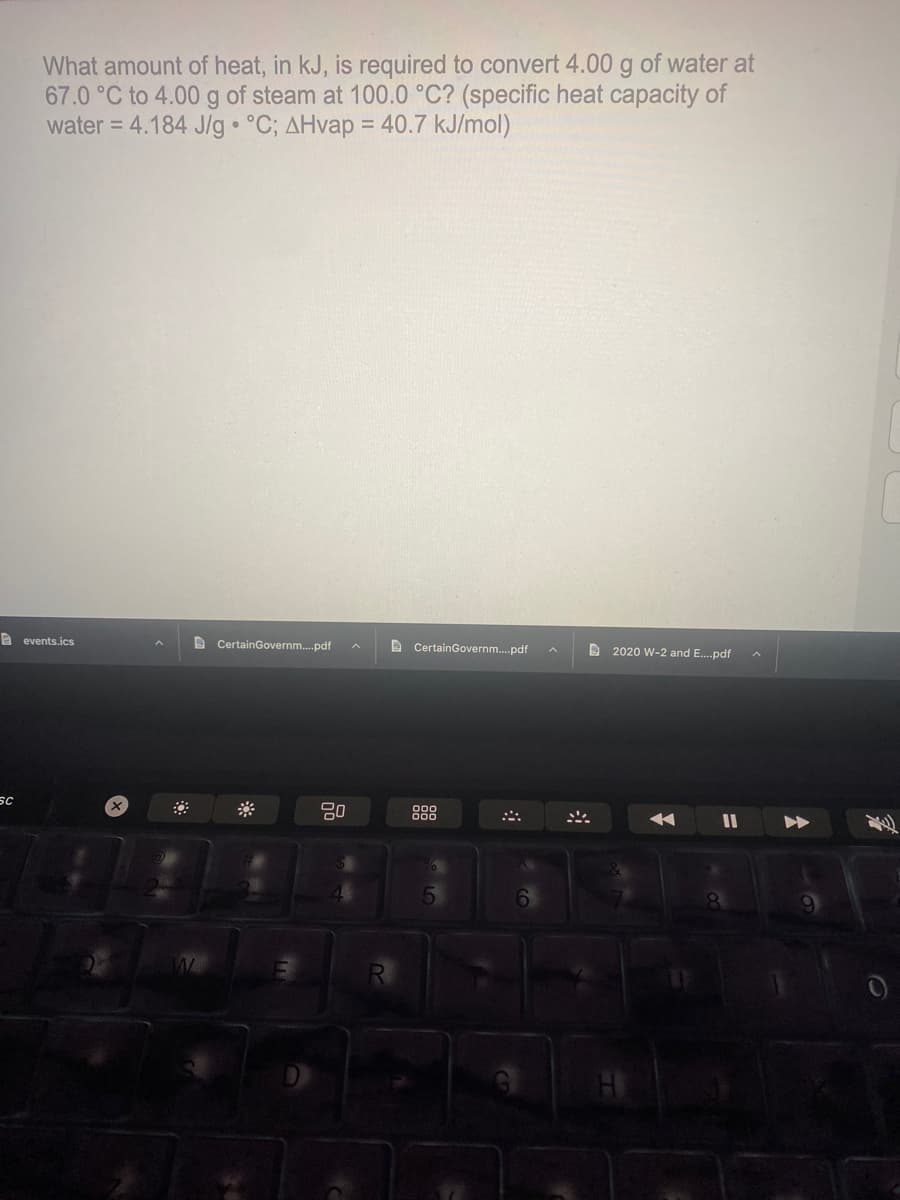
Transcribed Image Text:What amount of heat, in kJ, is required to convert 4.00 g of water at
67.0 °C to 4.00 g of steam at 100.0 °C? (specific heat capacity of
water = 4.184 J/g • °C; AHvap = 40.7 kJ/mol)
A events.ics
D CertainGovernm.pdf
D CertainGovernm.pdf
D 2020 W-2 and E.pdf
sc
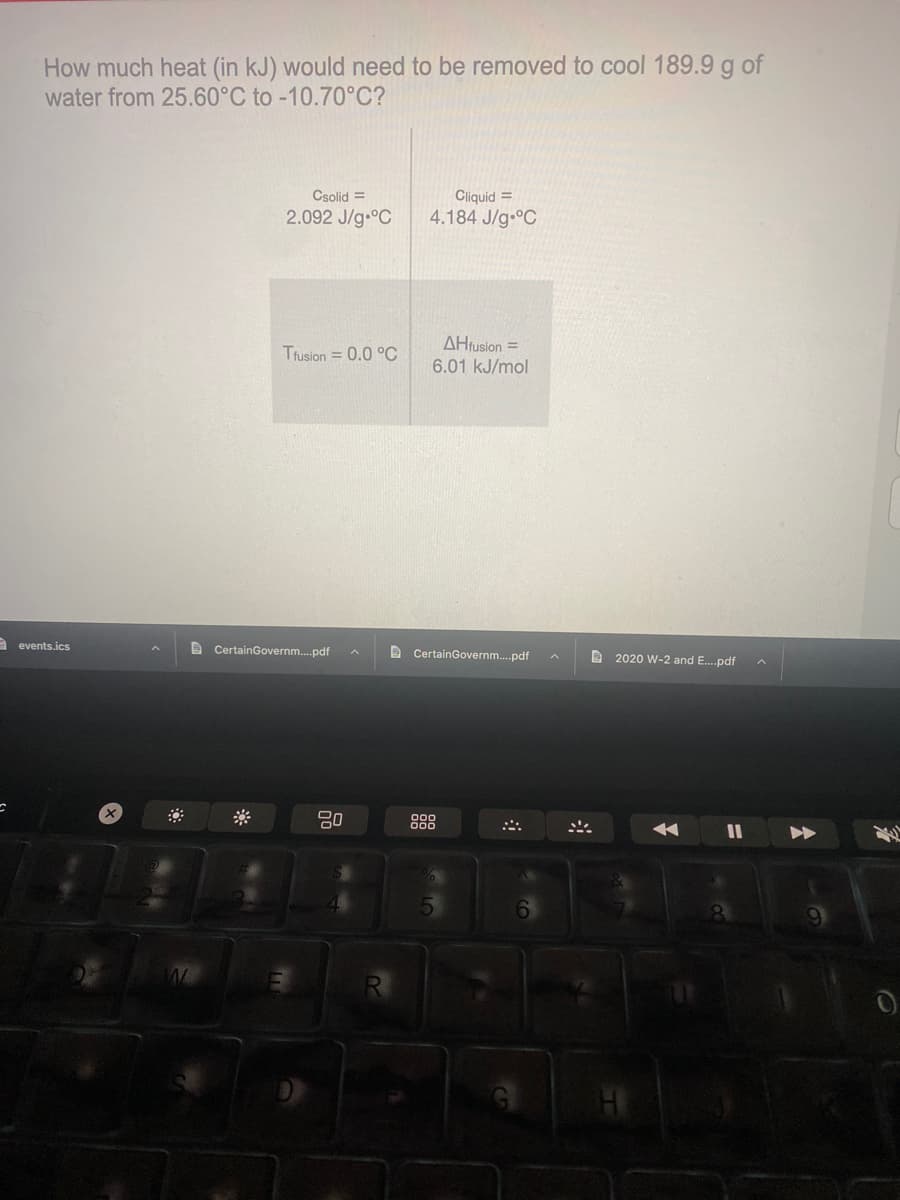
Transcribed Image Text:How much heat (in kJ) would need to be removed to cool 189.9 g of
water from 25.60°C to -10.70°C?
Csolid =
Cliquid =
2.092 J/g-°C
4.184 J/g C
AHrusion =
Trusion = 0.0 °C
6.01 kJ/mol
A events.ics
D CertainGovernm.pdf
D CertainGovernm.pdf
D 2020 W-2 and E.pdf
888
Expert Solution
Step 1
Heat required to raise the temperature of 4g water from 670C to 1000C
Q1 = mc∆T
m= 4g
c = specific heat capacity of water = 4.184 J/g0C
∆T = 100-67 = 330C
Latent heat of vaporisation is the amount of energy required to convert unit mass of water into steam.
∆Hv = 40.7 kJ/mol
Q2 = m*∆Hv
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co