What are the possible magnetic quantum numbers (m2) associated with each indicated value of €? When l = 2, mı = O -2,2 O -2, –1,0, 1, 2 O 0, 1, 2 -2, –1,1,2 When l = 5, mų = O -5, 5 O 0,1, 2, 3, 4, 5 O -5, –4, –3, -2, –1,0, 1, 2, 3, 4, 5 O -5, -4, -3, –2, –1, 1, 2, 3, 4, 5 Calculate the wavelength, in nanometers, of the spectral line produced when an electron in a hydrogen atom undergoes the transition from the energy level n = 3 to the level n = 2.
What are the possible magnetic quantum numbers (m2) associated with each indicated value of €? When l = 2, mı = O -2,2 O -2, –1,0, 1, 2 O 0, 1, 2 -2, –1,1,2 When l = 5, mų = O -5, 5 O 0,1, 2, 3, 4, 5 O -5, –4, –3, -2, –1,0, 1, 2, 3, 4, 5 O -5, -4, -3, –2, –1, 1, 2, 3, 4, 5 Calculate the wavelength, in nanometers, of the spectral line produced when an electron in a hydrogen atom undergoes the transition from the energy level n = 3 to the level n = 2.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 131QRT
Related questions
Question
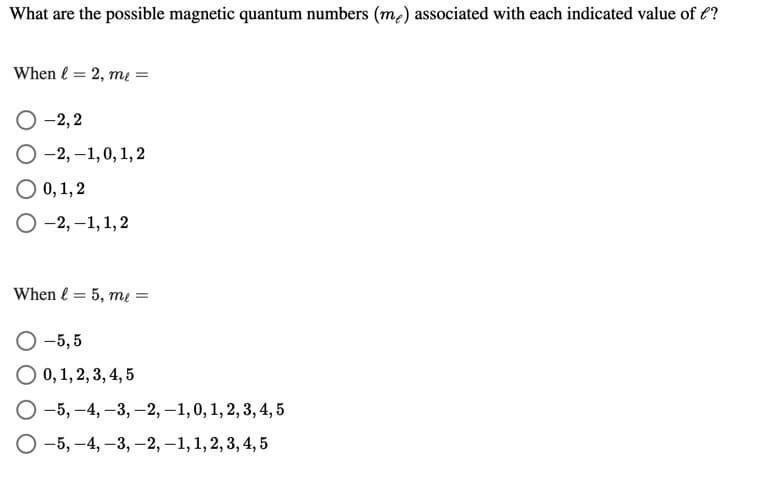
Transcribed Image Text:What are the possible magnetic quantum numbers (m2) associated with each indicated value of €?
When l = 2, mı =
O -2,2
O -2, –1,0, 1, 2
O 0, 1, 2
-2, –1,1,2
When l = 5, mų =
O -5, 5
O 0,1, 2, 3, 4, 5
O -5, –4, –3, -2, –1,0, 1, 2, 3, 4, 5
O -5, -4, -3, –2, –1, 1, 2, 3, 4, 5

Transcribed Image Text:Calculate the wavelength, in nanometers, of the spectral line produced when an electron in a hydrogen atom undergoes the
transition from the energy level n = 3 to the level n = 2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning