What distinguishes solids, liquid and gases is that O atoms move fastest in gases, slower in liquids and are rigidly connected in solids atoms move fastest in solids, slower in liquids and they are rigidly connected in gases the temperature of gases is always higher the temperature of solids is higher because they have more energy QUESTION 9 Two atoms of the same element O always have the same number of neutrons and protons O always have the same number of protons but can have different numbers of neutrons O always have the same number of electrons and protons O(a) and (c) QUESTION 10 We know that atoms of different elements combine to form molecules of compounds like table salt and water. What makes these bonds possible is Othe number of protons the number of electrons the electric force that attracts electrons and protons the electric force that attracts the neutrons from two different atoms
What distinguishes solids, liquid and gases is that O atoms move fastest in gases, slower in liquids and are rigidly connected in solids atoms move fastest in solids, slower in liquids and they are rigidly connected in gases the temperature of gases is always higher the temperature of solids is higher because they have more energy QUESTION 9 Two atoms of the same element O always have the same number of neutrons and protons O always have the same number of protons but can have different numbers of neutrons O always have the same number of electrons and protons O(a) and (c) QUESTION 10 We know that atoms of different elements combine to form molecules of compounds like table salt and water. What makes these bonds possible is Othe number of protons the number of electrons the electric force that attracts electrons and protons the electric force that attracts the neutrons from two different atoms
Chapter2: The Kinetic Theory Of Gases
Section: Chapter Questions
Problem 61P: A steel container of mass 135 g contains 24.0 g of ammonia, NH3, which has a molar mass of 17.0...
Related questions
Question
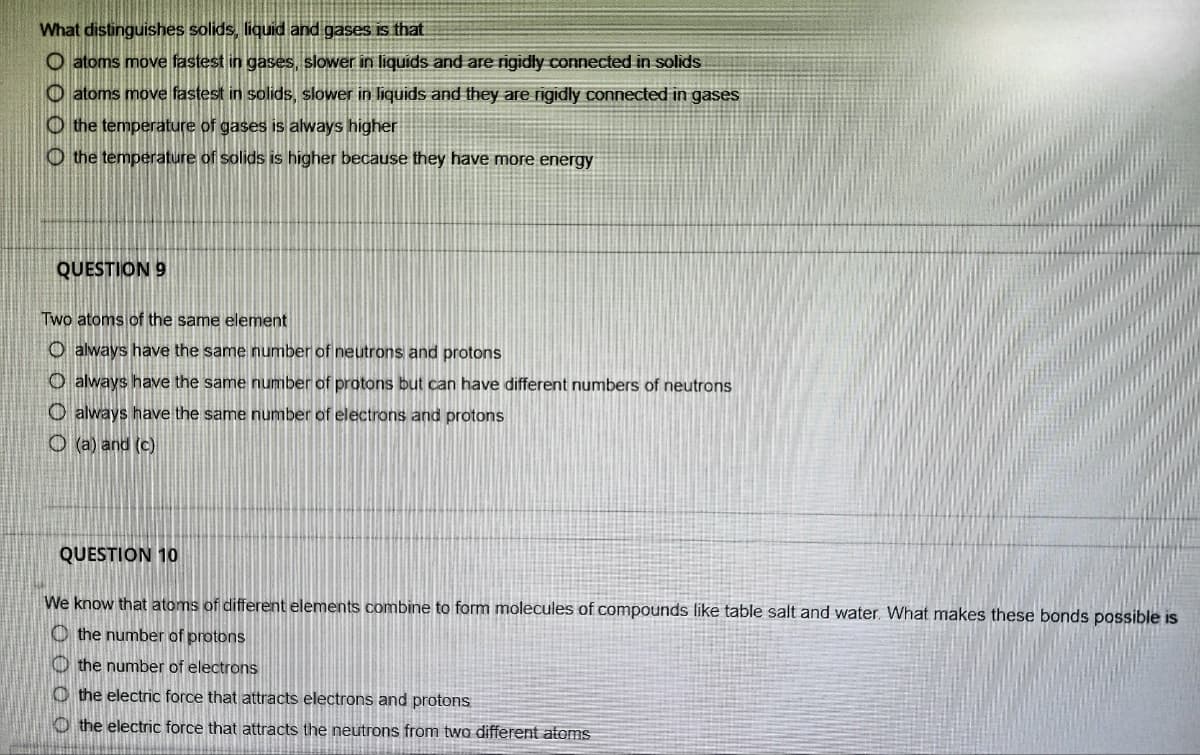
Transcribed Image Text:What distinguishes solids, liquid and gases is that
O atoms move fastest in gases, slower in liquids and are rigidly connected in solids
atoms move fastest in solids, slower in liquids and they are rigidly connected in gases
Othe temperature of gases is always higher
the temperature of solids is higher because they have more energy
QUESTION 9
Two atoms of the same element
O always have the same number of neutrons and protons
O always have the same number of protons but can have different numbers of neutrons
O always have the same number of electrons and protons
O(a) and (c)
QUESTION 10
We know that atoms of different elements combine to form molecules of compounds like table salt and water. What makes these bonds possible is
the number of protons
the number of electrons
the electric force that attracts electrons and protons
the electric force that attracts the neutrons from two different atoms
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning


Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning