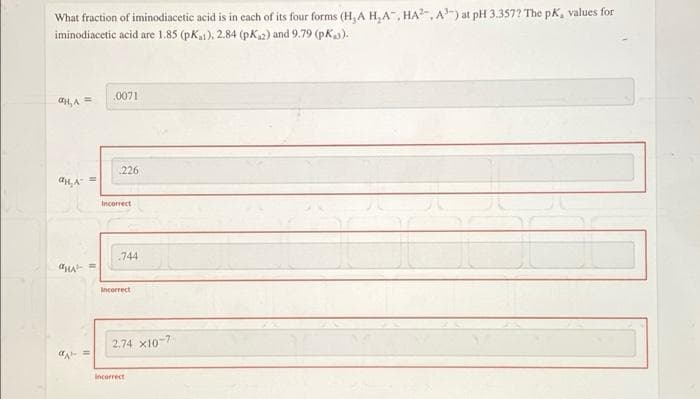
What fraction of iminodiacetic acid is in each of its four forms (H, A H,A, HA, A) at pH 3.357? The pK, values for iminodiacetic acid are 1.85 (pK), 2.84 (pk2) and 9.79 (pK).
Q: Which of the following is incorrect regarding electrodes of the first kind? O a. may depend on the p...
A: Electrode of the first kind is a simple metal electrode immersed in a solution containing its own io...
Q: Why is spin-pairing so common a features of bond formation (in the context of valence-bond theory)?
A: Spin pairing is two elctrons with opposite spins usually occupying the same orbital. A bond is for...
Q: Make a schematic diagram for the procedure below: B. % SO3 determination Dry the soluble sulfate s...
A:
Q: What is the pOH of a 0.084 M HNO3 solution.
A: Concentration of HNO3 is 0.084 M HNO3 ⇌ H+ + NO3- So, 1 HNO3 will give 1 H+
Q: Abuela asked Mirabel to perform an experiment on freezing point depression. The solution was prepare...
A: In part 3., mass of potassium nitrate is taken as mass of potassium chloride since it was mentioned ...
Q: The symmetry factor β was determined to be 0.4 for an activation controlled Ag deposition process. A...
A:
Q: The Keg for the reaction: A + B AB is 3 What is the Keg for 4 A + 4 B + 4 AB ? Submit Question
A: Keq = [product]÷[reactant]
Q: Draw the structure of 5-methyl-3-heptyne. Select Draw Rings More C H
A: Draw the structure of 5-methyl-3-heptyne = ?
Q: What is the molar solubility of Agl Ksp 8.3 x 1017 in the presence of 0.05 M KI? O 2.34 x 10^(-11) M...
A:
Q: IZ What is the IUPAC name for the compound shown? CH3 H3C The IUPAC name is:
A:
Q: N но What is the chemical formula for the limiting reactant in the reaction shown? chemical formula:...
A:
Q: Black smokers are found in the depths of the oceans. Thinking that the conditions in these smokers m...
A: 2 CH3SH + CO -----> CH3 COS CH3 + H2S If CO is taken in excess the theoretical yield depends on ...
Q: Calculatn Suppose a 500. mL flask is filled with 1.5 mol of H, and 0.80 mol of HI. The following rea...
A: We have to calculate the equilibrium concentration of Hydrogen gas.
Q: ness. 5. The second law of Thermodynamics states that for any spontaneous process, there is a net in...
A: The second law of thermodynamics predicts the spontaneous nature of a process. According to this law...
Q: Calculate the volume, in L, of water that must be added to dilute 24.6 mL of 10.6 M HCI to 0.105 M H...
A:
Q: on 1 of 3 The decomposition of N,O, can be described by the equation 2N,0, (soln) 4 NO, (soln) + O,(...
A:
Q: 0/5 O ACIDS AND BASES Calculating the pH at equivalence of a titration A chemist titrates 190.0 mL o...
A:
Q: Question 28. its levels are lower (in the blood stream) if you eat unsaturated lipids. Galactose isa...
A: Unsaturated Lipids Lowers the Value oF Bad cholesterol in the blood.
Q: Consider dissolving the barium nitrate in nitric acid HNO3 , a strong acid (it reacts completely wit...
A: Consider dissolving the barium nitrate in nitric acid HNO3 , a strong acid (it reacts completely wit...
Q: 1. If 15.4 g of Cu react with excess nitric acid, how many grams of nitrogen monoxide are produced? ...
A: To find grams of nitrogen monoxide formed, • Convert the given mass of Cu to moles. • Find out the...
Q: You have reached copper chloride solution with Zn metal to precipitate out all the Cu in its element...
A: Zn is more reactive metal then Copper.
Q: 2. Write balanced net ionic equations describing each of the following reactions: Precipitation of t...
A: 1. Precipitation of tin(IV) sulfide with H2S Sn4+ (aq) + 2 H2S (aq) → SnS2 (s) + 2H+ (aq) Here SnS...
Q: The following data are for the gas phase decomposition of sulfuryl chloride at 600 K. S02Cl2(g) → SO...
A:
Q: of 10 Draw the major organic product for the reaction shown. Select Draw Rings More Erase C H Br 1. ...
A: The given reaction is an example of an witting reaction . Witting reaction : The reaction in which ...
Q: *OCH3 Br DMSO HO
A: Charged species of alcohol(methoxide -OCH3) are strong nucleophiles. So which undergoes via SN2 reac...
Q: Name the highlighted chemical group in each molecule. Lewis structure name of highlighted group H Н—...
A:
Q: The decomposition of N,O, can be described by the equation 2N,0,(soln) → 4 NO,(soln) + 0,(g) Conside...
A: Answer: Given reaction is thermal decomposition reaction of N2O5 2N2O5(soln)→4NO2(soln)+O2(g) The ...
Q: Calculate the magnesium ion molarity for a solution which has magnesium ion concentration of 176.4 p...
A: Concentration of magnesium ion = 176.4 ppm Molarity of this solution = ?
Q: Determine the percent composition of BF3 if a 75.0 sample of BF3 decomposes and produces 63.1 g of f...
A:
Q: In Fehling's reaction heat is ? a)Reducer b) Catalyser c)Substrate explain the answer
A:
Q: Predict the location where a new substituent will be added when this aromatic compound undergoes an ...
A:
Q: Which of the following statements about the n-electron system of a linear conjugated organic molecul...
A: According to the molecular orbital theory the atomic orbitals combine to form molecular orbital. The...
Q: HgCl2(s)/Hg(1) CO2(g)/CH3OH | Write the redox half-equation then deduce the expression of the Nernts...
A: Given: To write half cell equations and deduce expression of Nernst law
Q: QUESTION 4 Predict the major product for the following reaction: Br .CH CH2 1.(CH,),N: 2. КОН H3C *C...
A:
Q: The following mechanism has been proposed for the conversion of tert-butyl bromide to tert-butyl alc...
A:
Q: Reagent Concentration (Molecular weight) Amount Needed Bicine 5.00 mM (163.17) NaCI 500.0 mM (58.44)...
A: Amount needed calculated by the concentration
Q: The trans isomer of the compound below is more stable than its cis isomer. True or false?
A: Stereochemistry is branch of chemistry in which we deal with arrangement of atoms in molecules.
Q: Use the standard reduction potentials at 25° C in Table 18.1 in Tro, Fridgen and Shaw, and calculate...
A:
Q: How to tell if a molecule is polar or non-polar without electronegativity?
A: Can be explained by using symmetry....
Q: . What is reactant selectivity in zeolite catalysis?
A: What is reactant selectivity in zeolite catalysis?
Q: Determine the percentage of aspirin (acetylsalicylic acid) recovered from your commercial aspirin ta...
A: Determine the percentage of aspirin ( acetylsalicylic acid)recovered from your commercial aspirin ta...
Q: What is the concentration of H* in a 2.5 M HCI (100 mL) and 0.1 M HBr (100 mL) solution? 0 1.8 М O 2...
A: Molarity of HCl = Molarity of H+ ion Molarity of HBr = Molarity of H+ ion Total Mole of H+ ion = M...
Q: Suppose that you have 175 mL of a buffer that is 0.500 M in both hydrofluoric acid (HF) and its conj...
A:
Q: Assuming the above reaction is exothermic in the forward direction (toward the product side), predic...
A:
Q: carefully open it. Observe 2. Drink a little after you opened and leave it for open for 20-30 mins. ...
A: Carbonated drinks:
Q: What does the number in a filter paper's brand represent?
A:
Q: A solution is prepared by adding 100.0 mL of a 0.135 M HCI solution to 100.0 mL of a 0.066 M NAOH so...
A: Volume of HCl=100.0mL Concentration of HCL=0.135M Volume of NaOH=100mL Concentration of NaOH=0.066...
Q: ) What is the name of the compound below? a) 3-oxopentanoic acid b) 1-acetopentanone c) acetopentano...
A: Here we have to write the IUPAC name of the following componund whose structure is given.
Q: 5. Tropone (A) is much stronger base (conjugate acid more stabilized) than the open chain, conjugate...
A:
Q: Activity 2 Assignment Lesson 9 - Moles, Mass, and Number of Molecules Module 3: Chemical Calcula...
A: Mole: A mole is defined as total 6.02214076 × 1023 unit of some chemical intity. It can be atoms, mo...
Step by step
Solved in 6 steps

- Experimental observations show that thiocyanate (SCN- ), the conjugate base of thiocyanic acid (pKa = 1.1 at 25 °C), is quite soluble in neutral water at pH > 2. In the space provided below, explain this experimental observation. That is, why does SCN– readily dissolve in water when the pH > 2?Glutamic acid is a naturally occurring α-amino acid that contains acarboxy group in its R group side chain. (Glutamic acid isdrawn in its neutral form with no charged atoms, a form that does not actually exist at any pH.) a.) What form of glutamic acid exists at pH = 1?b.) If the pH is gradually increased, what form of glutamic acid exists afterone equivalent of base is added? After two equivalents? After threeequivalents?c.) Propose a structure of monosodium glutamate, the common flavorenhancer known as MSG.When BF3 behaves as a Lewis acid, the stability of the adducts with (CH3)2O, (CH3)2S, and (CH3)2Se decreases in the order in which the Lewis bases are listed. However, when the Lewis acid is B(CH3)3, the most stable adduct is that formed with (CH3)2S and the adducts with (CH3)2Se ad (CH3)2O are about equally stable. Explain the difference in stability of the adducts
- What percent of acetic acid is present in the acidic form at pH 5.0, assuming a pKa of 4.8?A typical amino acid with one amino group and one carboxylicacid group, such as serine, can exist in water in several ionic forms. (a) Suggest the forms of the amino acidat low pH and at high pH. (b) Amino acids generally havetwo pKa values, one in the range of 2 to 3 and the other inthe range of 9 to 10. Serine, for example, has pKa values of2.19 and 9.21. Using species such as acetic acid and ammoniaas models, suggest the origin of the two pKa values.(c) Glutamic acid is an amino acid that has threepKa's: 2.10, 4.07, and 9.47. Draw the structure of glutamicacid, and assign each pKa to the appropriate part of themolecule. (d) An unknown amino acid is titrated withstrong base, producing the following titration curve.Which amino acids are likely candidates for the unknown?A typical amino acid with one amino group and one carboxylic acid group, such as serine can exist in water in several iconic forms. Suggest the forms of the amino acid at low pH and at a high pH. Amino acids generally have two pKa values, one in the range of 2 to 3 and the other in the range of 9 to 10. Serene, for example, has pKa values of 2.91 and 9.21. Using species such as acetic acid and ammonia as models, suggest the origin of the two pKa values. Glutamic acid is an amino acid that has three pKa’s: 2.10, 4.07, and 9.47. Draw the structure of glutamic acid and assign each pKa to the appropriate part of the molecule. An unknown amino acid is titrated with strong base, producing the following titration curve. Which amino acid are likely candidates for the unknown?
- Valine has pKa's of 2.286 and 9.719. Estimate the fractional composition of Valine in the -1 form at pH=6.Tyrosine is an amino acid whose side chain has a pKa of 10.1. At pH 7, what protonation form would you expect to find it in?PICTURE ATTACHED Glutaric Acid is a diprotic organic acid with a molecular formula of H2C5H6O4, and a molecular weight of 132.12 g/mol. The stepwise acid dissociation reactions and the Ka values are given below. An aqueous solution is prepared by dissolving 1.02 g of glutaric acid in water to form 500.0 ml of solution. Calculate the pH, [H2C5H6O4], [HC5H6O4–], and[C5H6O42–] after the solution has reached equilibrium.

