Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter3: Molecules, Moles, And Chemical Equations
Section: Chapter Questions
Problem 3.108PAE: 3.108 As chip speeds increase, the width of the interconnects described in Problem 3.107 must be...
Related questions
Question
Q: What is the concentration of copper in mM?
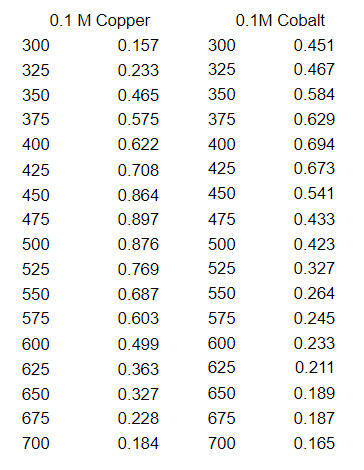
Transcribed Image Text:0.1 M Copper
0.1M Cobalt
300
0.157
300
0.451
325
0.233
325
0.467
350
0.465
350
0.584
375
0.575
375
0.629
400
0.622
400
0.694
425
0.708
425
0.673
450
0.864
450
0.541
475
0.897
475
0.433
500
0.876
500
0.423
525
0.769
525
0.327
550
0.687
550
0.264
575
0.603
575
0.245
600
0.499
600
0.233
625
0.363
625
0.211
650
0.327
650
0.189
675
0.228
675
0.187
700
0.184
700
0.165
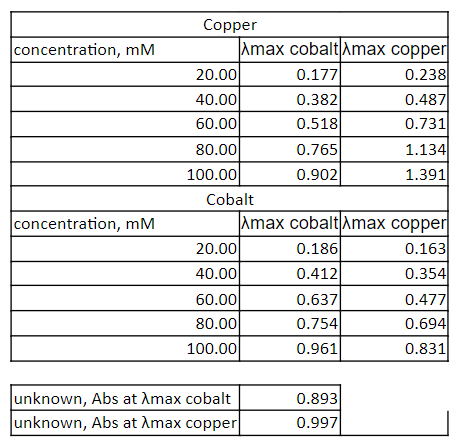
Transcribed Image Text:Copper
concentration, mM
Amax cobaltAmax copper
20.00
40.00
60.00
0.177
0.238
0.487
0.731
0.382
0.518
80.00
0.765
1.134
100.00
0.902
1.391
Cobalt
concentration, mM
Amax cobalt Amax copper
20.00
0.186
0.163
40.00
0.412
0.354
0.477
0.694
0.831
60.00
0.637
80.00
0.754
100.00
0.961
unknown, Abs at Amax cobalt
unknown, Abs at Amax copper
0.893
0.997
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning