What is the effect of a nonvolatile solute on the vapor pressure of a liquid?
The non-volatile solute is the type of solute that does not evaporate on its own. That is, the boiling point of this type of solute is comparatively high.
Vapor pressure is the pressure caused by the vapor molecules of the gas in a container.
The vapor pressure of the pure solvent (water) is constant. But when the non-volatile solute is added to the pure solvent, the vapor pressure lowers.
This phenomenon is known as the lowering of vapor pressure. The relative lowering of vapor pressure is one of the colligative properties of the solution.
The result for lowering of vapor pressure is, the addition of the solute particle in the pure solvent. When the solute is added the number of solvent molecules escaping as vapors now becomes less. Because of which the vapor pressure of the pure solvent is now reduced.
According to Raoult's law, lowering of vapor pressure can be given as:
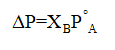
Where,

Step by step
Solved in 3 steps with 2 images









