What is the experimental molar mass (that is, calculated from the data given and not taken from the periodic table) of magnesium if 0.0269 grams of magnesium generates 26.20 mL of hydrogen at 22.7 °C with a hydrogen partial pressure of 776.8 torr? Note that the partial pressure of water vapor has already been subtracted from the total pressure. 19.1 g/mol 24.4 g/mol 22.4 g/mol 26.6 g/mol 26.5 g/mol
What is the experimental molar mass (that is, calculated from the data given and not taken from the periodic table) of magnesium if 0.0269 grams of magnesium generates 26.20 mL of hydrogen at 22.7 °C with a hydrogen partial pressure of 776.8 torr? Note that the partial pressure of water vapor has already been subtracted from the total pressure. 19.1 g/mol 24.4 g/mol 22.4 g/mol 26.6 g/mol 26.5 g/mol
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter9: Gases
Section: Chapter Questions
Problem 95E: A 1-L sample of CO initially at STP is heated to 546 K. and its volume is increased to 2 L. (a) What...
Related questions
Question
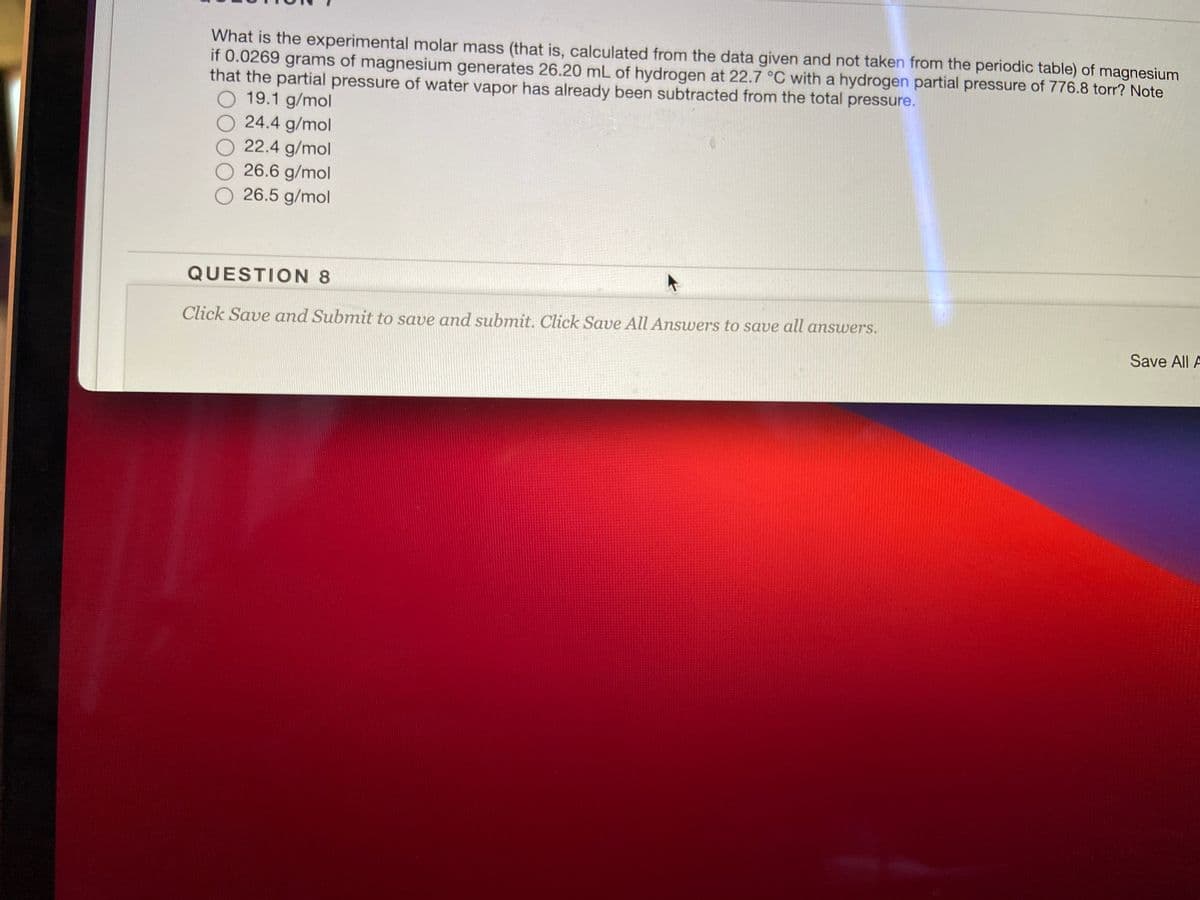
Transcribed Image Text:What is the experimental molar mass (that is, calculated from the data given and not taken from the periodic table) of magnesium
if 0.0269 grams of magnesium generates 26.20 mL of hydrogen at 22.7 °C with a hydrogen partial pressure of 776.8 torr? Note
that the partial pressure of water vapor has already been subtracted from the total pressure.
O 19.1 g/mol
24.4 g/mol
22.4 g/mol
26.6 g/mol
26.5 g/mol
QUESTION 8
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
Save All A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning