Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.34QAP
Related questions
Question
Chemistry
- What is the identity of each of your unknowns (e.g.
aldehyde , alcohol, etc.)? Provide support for your choice.
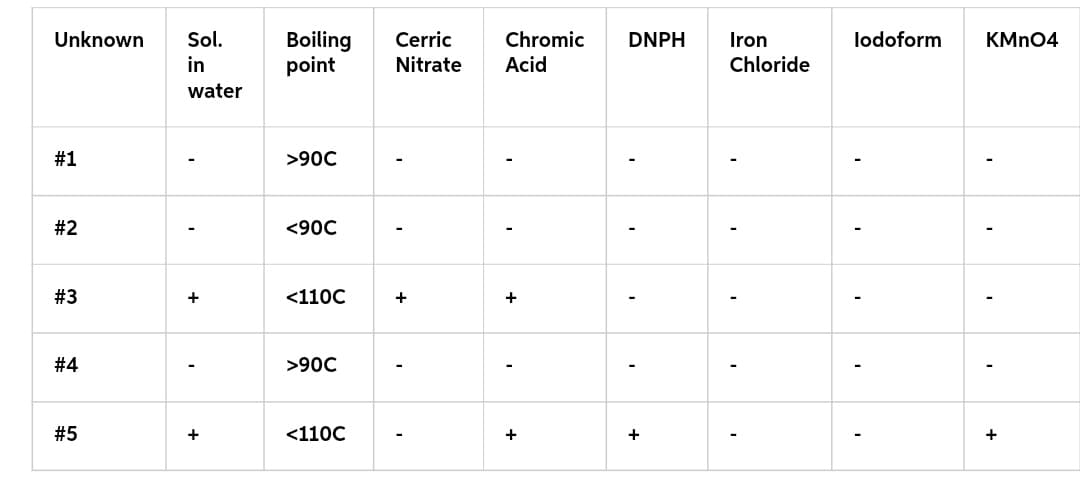
Transcribed Image Text:Unknown Sol.
in
water
#1
#2
#3
#4
#5
I
+
+
Boiling
point
>90C
<90C
<110C
>90C
<110C
Cerric
Nitrate Acid
+
Chromic
+
+
DNPH
I
I
+
Iron
Chloride
I
lodoform KMnO4
I
'
+
Expert Solution
Step by step
Solved in 9 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

