What is the kinetic energy of the emitted electrons when cesium is exposed to UV rays of frequency 1.90×1015Hz? Express your answer in joules to three significant figures.
What is the kinetic energy of the emitted electrons when cesium is exposed to UV rays of frequency 1.90×1015Hz? Express your answer in joules to three significant figures.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 34QRT: Spectroscopists have observed He+ in outer space. This ion is a one-electron species like a neutral...
Related questions
Question
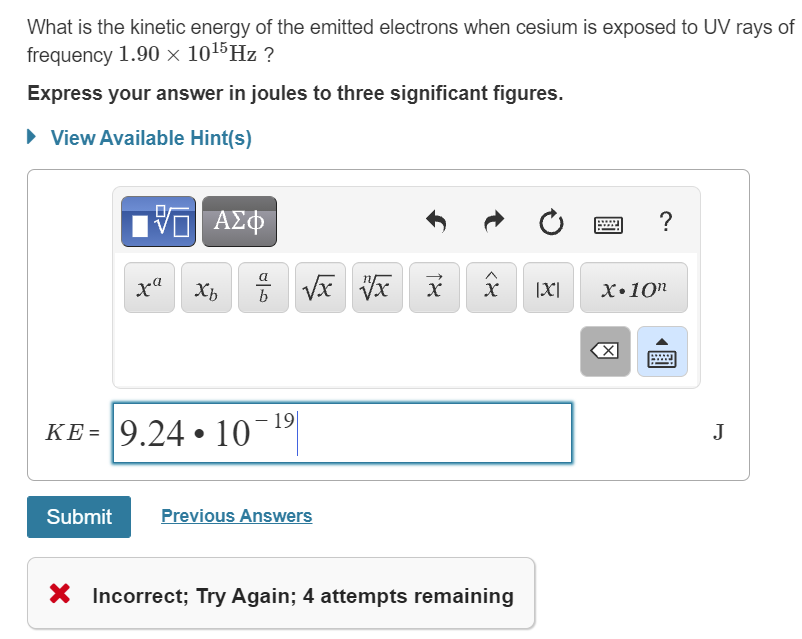
What is the kinetic energy of the emitted electrons when cesium is exposed to UV rays of frequency 1.90×1015Hz?
Express your answer in joules to three significant figures.
Transcribed Image Text:What is the kinetic energy of the emitted electrons when cesium is exposed to UV rays of
frequency 1.90 × 1015 Hz ?
Express your answer in joules to three significant figures.
• View Available Hint(s)
?
a
|X|
х.10п
– 19|
KE = 9.24 • 10¯1
J
Submit
Previous Answers
X Incorrect; Try Again; 4 attempts remaining
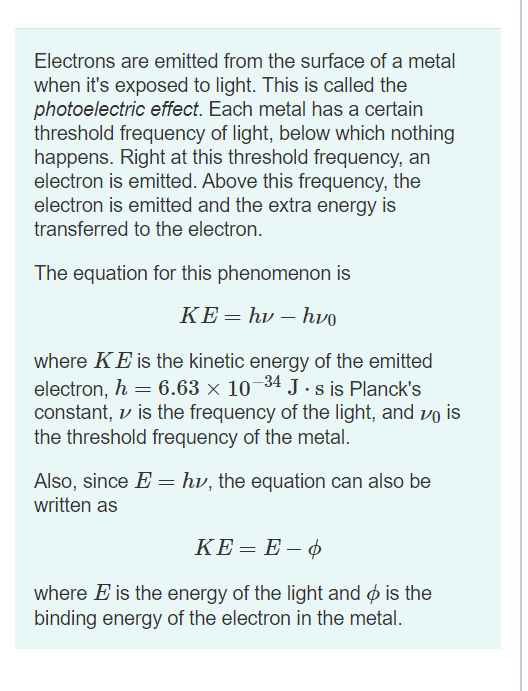
Transcribed Image Text:Electrons are emitted from the surface of a metal
when it's exposed to light. This is called the
photoelectric effect. Each metal has a certain
threshold frequency of light, below which nothing
happens. Right at this threshold frequency, an
electron is emitted. Above this frequency, the
electron is emitted and the extra energy is
transferred to the electron.
The equation for this phenomenon is
ΚΕhv-hvo
where KE is the kinetic energy of the emitted
electron, h = 6.63 × 10¬34 J. s is Planck's
constant, v is the frequency of the light, and vo is
the threshold frequency of the metal.
Also, since E = hv, the equation can also be
written as
KE= E – ¢
where E is the energy of the light and ø is the
binding energy of the electron in the metal.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning