What is the mass of 2.5 moles of zinc chloride (ZnCl₂)? bign Li Be Na Mg 3 Rb kidn 341 g Fr Ra Paslan 276 g Ca Sc Ti Sode 47.8 Gen Ba 198.3 g 383 g 136.4 g 297.3 g 4 IVB 45-103 Activid Hf Pan VB 50.342 Ta Metal Taide 104 105 Rf "Y Zr Nb Mo Tc Ru Rh Pd Min M Duter Bod Pisti 10042 W Beg Periodic Table of the Elements Ac Th Pa ATH VIB 781 24 4427 +29 Cr Mn Fe Co Ni Ovost tot 15 T A Symbol 9 VII Metal Re Os Ir F 10 Rad 11 S Med Ag Shet 12 IB 105 107 100 109 110 111 112 Db Sg Bh Hs Mt Ds Rg Cn Nh Cu Zn Ga Catedr Pt Au Hg DE Heleem BCN 14 MA Np Pu Am Cm Bk Cf Es tete STEAIA Noble TI Pb Th Si P 15 WA Ge As Se Ak 24302 113 114 115 Fe Sn Sb Te Min 1170 Bi Lantharide 16 VIA Mc La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb www ten interne S STATE 17 VIA JA O F Ne www www CI CHO Br bo TRERE de Po At He B 18 VRIA PA UN ARX Ar Kr Xe 7 szkesse 115 iškrone Ts Og Lu Rn Fm Md No Lr
What is the mass of 2.5 moles of zinc chloride (ZnCl₂)? bign Li Be Na Mg 3 Rb kidn 341 g Fr Ra Paslan 276 g Ca Sc Ti Sode 47.8 Gen Ba 198.3 g 383 g 136.4 g 297.3 g 4 IVB 45-103 Activid Hf Pan VB 50.342 Ta Metal Taide 104 105 Rf "Y Zr Nb Mo Tc Ru Rh Pd Min M Duter Bod Pisti 10042 W Beg Periodic Table of the Elements Ac Th Pa ATH VIB 781 24 4427 +29 Cr Mn Fe Co Ni Ovost tot 15 T A Symbol 9 VII Metal Re Os Ir F 10 Rad 11 S Med Ag Shet 12 IB 105 107 100 109 110 111 112 Db Sg Bh Hs Mt Ds Rg Cn Nh Cu Zn Ga Catedr Pt Au Hg DE Heleem BCN 14 MA Np Pu Am Cm Bk Cf Es tete STEAIA Noble TI Pb Th Si P 15 WA Ge As Se Ak 24302 113 114 115 Fe Sn Sb Te Min 1170 Bi Lantharide 16 VIA Mc La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb www ten interne S STATE 17 VIA JA O F Ne www www CI CHO Br bo TRERE de Po At He B 18 VRIA PA UN ARX Ar Kr Xe 7 szkesse 115 iškrone Ts Og Lu Rn Fm Md No Lr
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 89QAP: By x-ray diffraction it is possible to determine the geometric pattern in which atoms are arranged...
Related questions
Question
100%
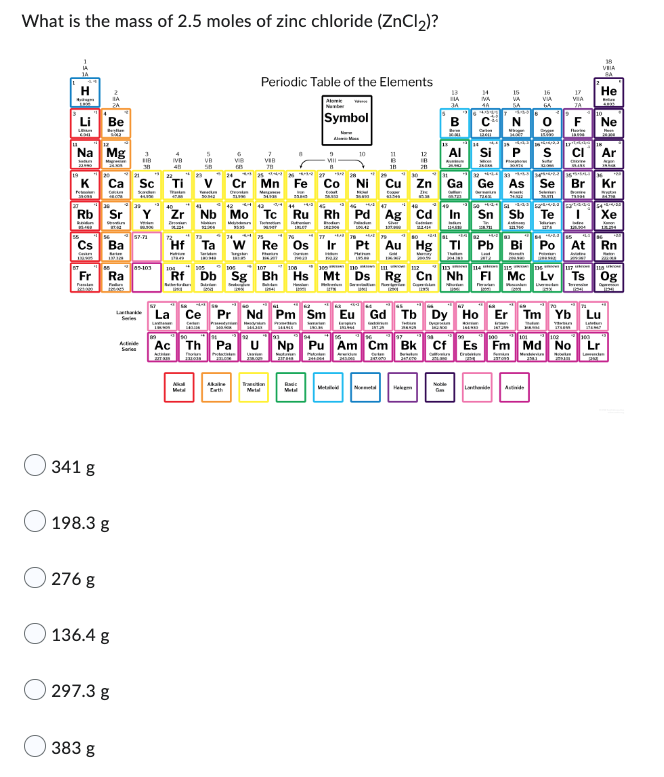
Transcribed Image Text:What is the mass of 2.5 moles of zinc chloride (ZnCl₂)?
- I
Na Mg
Be
Bezdice
Rb Sr
kad
Ges
341 g
Fr Ra
Plassian
Fadders
276 g
Ca Sc Ti
Ba
Kai
198.3 g
383 g
136.4 g
297.3 g
118
85-103
Series
IVB
VB
58
www
50.342
72 73
Hf
Ta
G
Periodic Table of the Elements
Alic
Nawber
Symbol
7
VIB
78
24. * 25 26 227 228
Ac Th Pa
3/4 44 1419 45
Zr Nb Mo Tc Ru Rh Pd
Nun
Puter
da
9
Mill
Mn Fe Co Ni
Cout
10
476
***70
Re Os Ir Pt
Akaike Trandan
Metal
B
Radc
12
18
13
BA
34
B
18
Cu Zn Ga
14
IMA
Helegm
15
VA
5A
N
www
16
VIA
0
15000
Lanthande
Site
In Sn Sb Te
17
VIA
JA
TID
104
Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og
S
Sirian
18
VEIA
BA
Ge As Se Br Kr
TATE
T
Ag
Xe
MA
Au Hg Tl Pb Bi Po At Rn
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
H
www
Despars
INDONE
THEM Dy
TIKSLA Latten
He
Ba
Ne
Np Pu Am Cm Bk Cf Es Fm Md No Lr
Lacan
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning