What is the minimum amount of ice at 0°C that must be added to the contents of a can of diet cola (340mL) to cool it down from 20.5°C to 0°C? Assume that the specific heat and density of the diet cola are the same as for water and that no heat is gained or lost to the surroundings. The latent heat of fusion of ice is 335J/g. Specific Heat of water 4.184 J/g.K, and Density of water is 1 g/mL.
What is the minimum amount of ice at 0°C that must be added to the contents of a can of diet cola (340mL) to cool it down from 20.5°C to 0°C? Assume that the specific heat and density of the diet cola are the same as for water and that no heat is gained or lost to the surroundings. The latent heat of fusion of ice is 335J/g. Specific Heat of water 4.184 J/g.K, and Density of water is 1 g/mL.
Chapter3: Magnetic Starters
Section: Chapter Questions
Problem 30SQ
Related questions
Question
What is the minimum amount of ice at 0°C that must be added to the contents of
a can of diet cola (340mL) to cool it down from 20.5°C to 0°C? Assume that the
specific heat and density of the diet cola are the same as for water and that no
heat is gained or lost to the surroundings. The latent heat of fusion of ice is 335J/g.
Specific Heat of water 4.184 J/g.K, and Density of water is 1 g/mL.
Transcribed Image Text:VoWiFi
43 1 7:44
53
3
A bartleby."
- Contact Support ioxtboon,ung,
Patrick
FRI AT 10:22 PM
Sir good eve po. Pwede mag pa
help dire
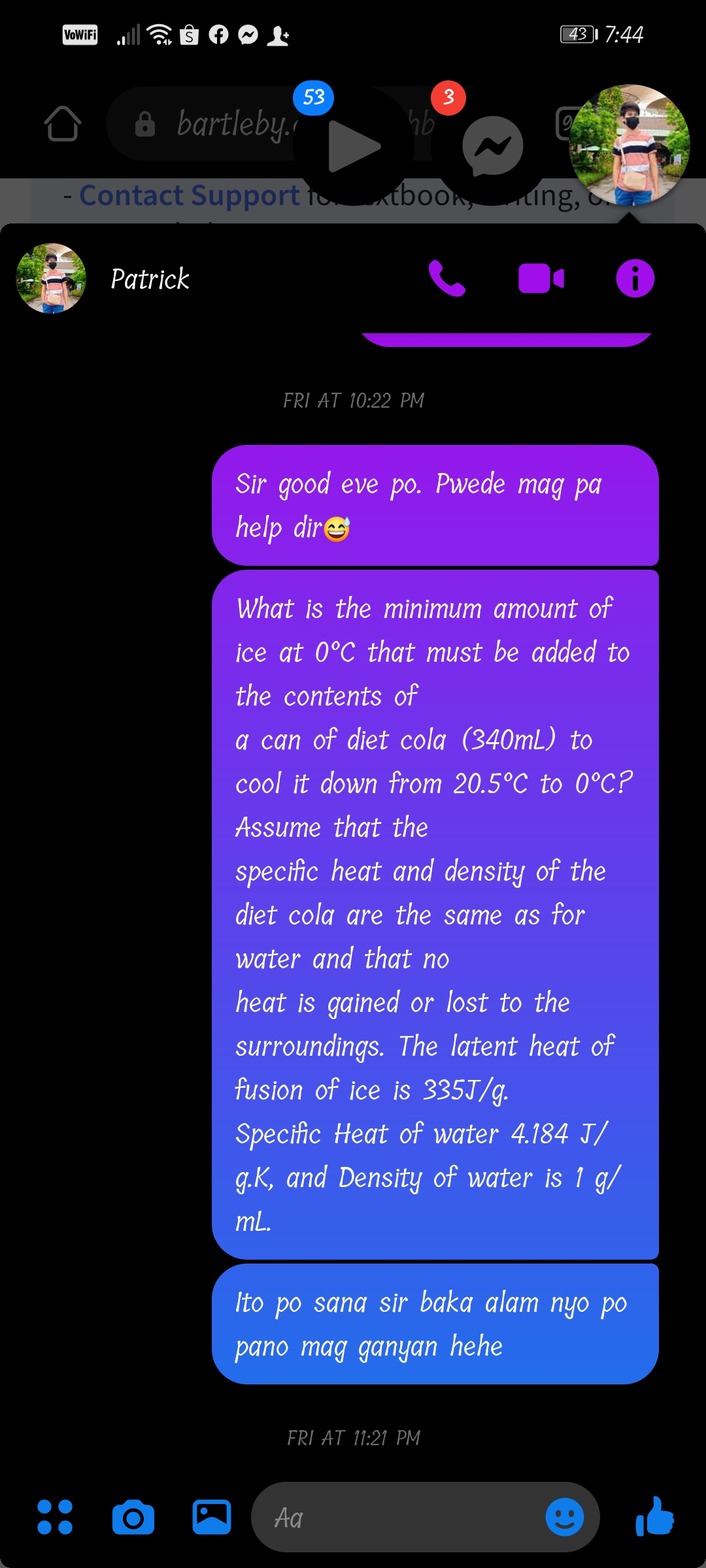
What is the minimum amount of
ice at 0°C that must be added to
the contents of
a can of diet cola (340mL) to
cool it down from 20.5°C to 0°C?
Assume that the
specific heat and density of the
diet cola are the same as for
water and that no
heat is gained or lost to the
surroundings. The latent heat of
fusion of ice is 335J/g.
Specific Heat of water 4.184 J/
g.K, and Density of water is 1 g/
mL.
Ito po sana sir baka alam nyo po
pano mag ganyan hehe
FRI AT 11:21 PM
Aa
Transcribed Image Text:VoWiFi
43 1 7:44
53
3
A bartleby."
- Contact Support ioxtboon,ung,
Patrick
FRI AT 10:22 PM
Sir good eve po. Pwede mag pa
help dire
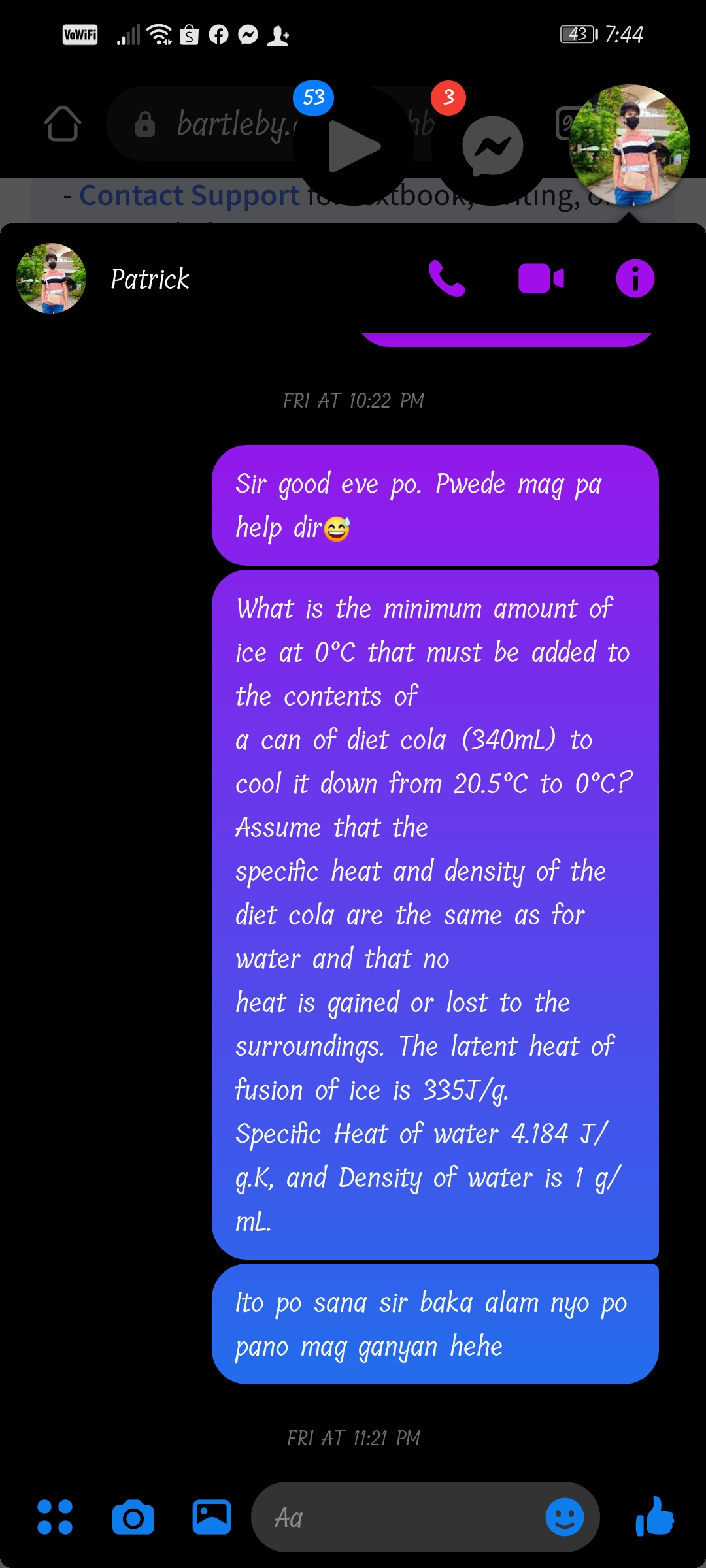
What is the minimum amount of
ice at 0°C that must be added to
the contents of
a can of diet cola (340mL) to
cool it down from 20.5°C to 0°C?
Assume that the
specific heat and density of the
diet cola are the same as for
water and that no
heat is gained or lost to the
surroundings. The latent heat of
fusion of ice is 335J/g.
Specific Heat of water 4.184 J/
g.K, and Density of water is 1 g/
mL.
Ito po sana sir baka alam nyo po
pano mag ganyan hehe
FRI AT 11:21 PM
Aa
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, electrical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you


Electricity for Refrigeration, Heating, and Air C…
Mechanical Engineering
ISBN:
9781337399128
Author:
Russell E. Smith
Publisher:
Cengage Learning

Power System Analysis and Design (MindTap Course …
Electrical Engineering
ISBN:
9781305632134
Author:
J. Duncan Glover, Thomas Overbye, Mulukutla S. Sarma
Publisher:
Cengage Learning


Electricity for Refrigeration, Heating, and Air C…
Mechanical Engineering
ISBN:
9781337399128
Author:
Russell E. Smith
Publisher:
Cengage Learning

Power System Analysis and Design (MindTap Course …
Electrical Engineering
ISBN:
9781305632134
Author:
J. Duncan Glover, Thomas Overbye, Mulukutla S. Sarma
Publisher:
Cengage Learning