What is the molecular weight for the gas whose specific heat are as follows? C, = 1.996 kJ/kgK, C,=1.507 kJ/kgK %3!
What is the molecular weight for the gas whose specific heat are as follows? C, = 1.996 kJ/kgK, C,=1.507 kJ/kgK %3!
Refrigeration and Air Conditioning Technology (MindTap Course List)
8th Edition
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Chapter50: Commercial, Packaged Rooftop, Variable Refrigerant Flow, And Variable Air Volume Systems
Section: Chapter Questions
Problem 18RQ: Explain how carbon dioxide levels can be used to estimate the occupancy level in a particular area...
Related questions
Question
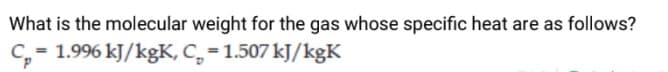
Transcribed Image Text:What is the molecular weight for the gas whose specific heat are as follows?
C, = 1.996 kJ/kgK, C, = 1.507 kJ/kgK
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning

Refrigeration and Air Conditioning Technology (Mi…
Mechanical Engineering
ISBN:
9781305578296
Author:
John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:
Cengage Learning