Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 29Q: When jump-starting a car with a dead battery, the ground jumper should be attached to a remote part...
Related questions
Question
what is the oxidizing agent and reducing agent in here
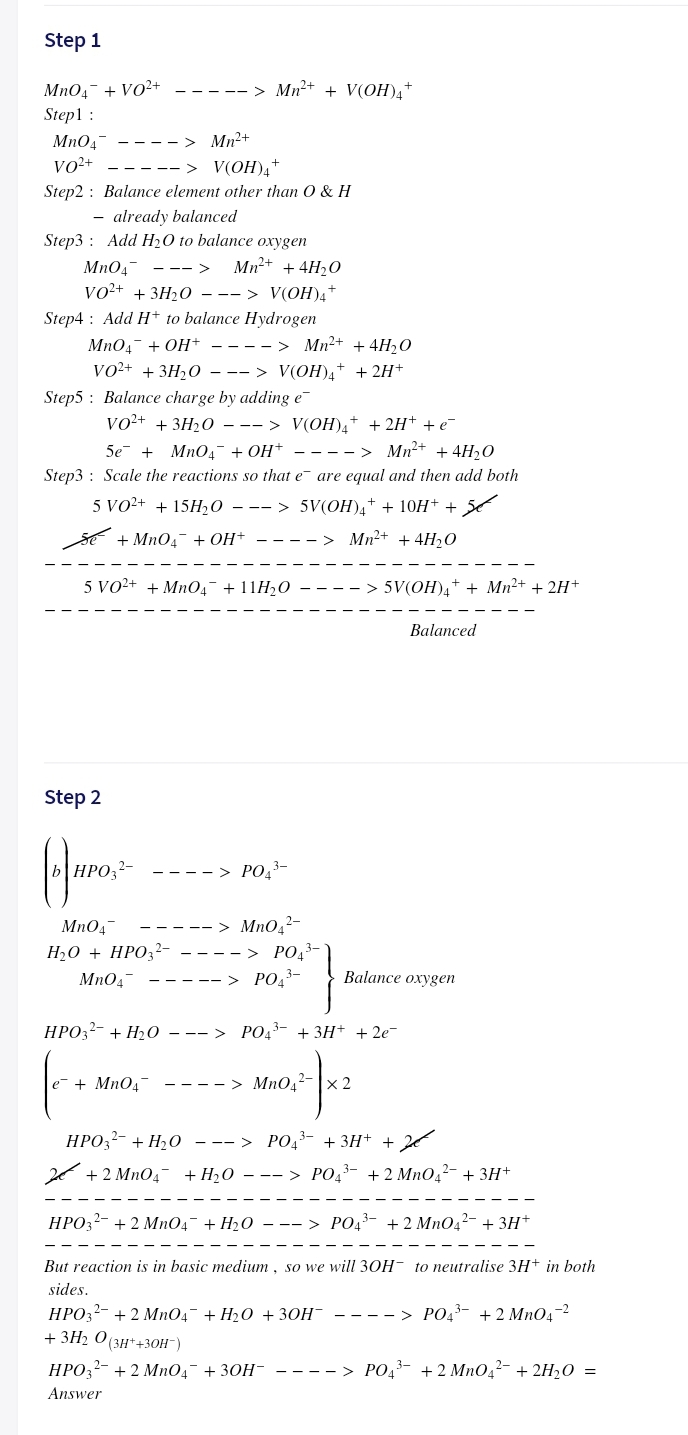
Transcribed Image Text:Step 1
MnO4- + VO2+
----- > Mn2+ + V(OH)A
Step1 :
Mn0,-
vo²+
- > Mn²+
-> V(OH)4+
Step2 : Balance element other than O & H
- already balanced
Step3 : Add H20 to balance oxygen
Mn04-
Mn+ + 4H2O
--->
Vo+ + 3H20 —--> V(OН),*
Step4 : Add H+ to balance Hydrogen
MnO4- + OH+ - ---> Mn2+ + 4H,0
VO+ + 3H,0 —--> V(OН)4* + 2H*
Step5 : Balance charge by adding e
vo²+ + 3H20 - -- > V(OH)4+ + 2H+ + e-
5e + MnO4¯ + OH+ - ---> Mn2+ + 4H20
Step3 : Scale the reactions so that e are equal and then add both
5 VO²+ + 15H½0
-- > 5V(OH)a+ + 10H+ +
sE + MnO4- + OH+
Mn2+ + 4H2O
---- >
5 VO2+ + Mn04¯ + 11H20
> 5V(OH),+ + Mn²+ + 2H+
Balanced
Step 2
HPO,?-
----> PO,-
MnO,2-
--> PO,3-
MnO4-
H20 + HPO32-
MnO4-
----- > PO,3-
Balance oxygen
НРОЗ- + H20о —--> РОд3- + ЗН+ + 2е-
е + MnO"
---- > MnO.- x 2
НРО,- + Н,о —--> РО,3- + ЗН+ + 2
+ 2 MnO4- +H,0 – -- > PO43- +2 MnOq?- + 3H+
HPO3?- + 2 MnO4- + H2O – –- > PO43- + 2 Mn042- + 3H*
But reaction is in basic medium, so we will 3OH- to neutralise 3H+ in both
sides.
HPO32- + 2 MnO4- + H20 + 30H¯ ---- > PO43- + 2 Mn04-2
+ 3H2 O (3H*+30H¯)
НРО,2- + 2 МnОд + 3ОН-
----> PO43- +2 Mn04²- + 2H20 =
Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
