What is the resulting molarity of LiOH after dilution (but BEFORE any possible chemical reaction) if (2.28x10^1) mL of (9.020x10^-1) M LİOH are mixed with (5.740x10^1) mL of (3.040x10^-1) M HCI?
What is the resulting molarity of LiOH after dilution (but BEFORE any possible chemical reaction) if (2.28x10^1) mL of (9.020x10^-1) M LİOH are mixed with (5.740x10^1) mL of (3.040x10^-1) M HCI?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 145CP: Consider an experiment in which two burets, Y and Z, are simultaneously draining into a beaker that...
Related questions
Question
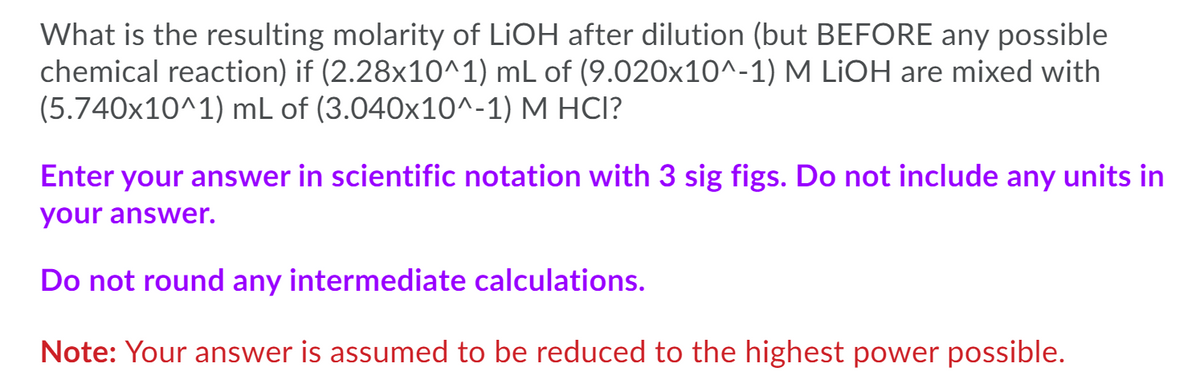
Transcribed Image Text:What is the resulting molarity of LIOH after dilution (but BEFORE any possible
chemical reaction) if (2.28x10^1) mL of (9.020x10^-1) M LİOH are mixed with
(5.740x10^1) mL of (3.040x10^-1) M HCI?
Enter your answer in scientific notation with 3 sig figs. Do not include any units in
your answer.
Do not round any intermediate calculations.
Note: Your answer is assumed to be reduced to the highest power possible.

Transcribed Image Text:What is the highest pH that could be used as an effective buffer with B (a generic
base) with Kp = (6.46x10^-10) and its chloride salt, HBCI?
Enter your answer in scientific notation with 3 sig figs. Do not include any units in
your answer.
Do not round any intermediate calculations.
Note: Your answer is assumed to be reduced to the highest power possible.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning