Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter10: Entropy And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 10.51PAE: 10.51 The combustion of acetylene was used in welder's torches for many years because it produces a...
Related questions
Question
PLease answer only number 2 with complete solution.
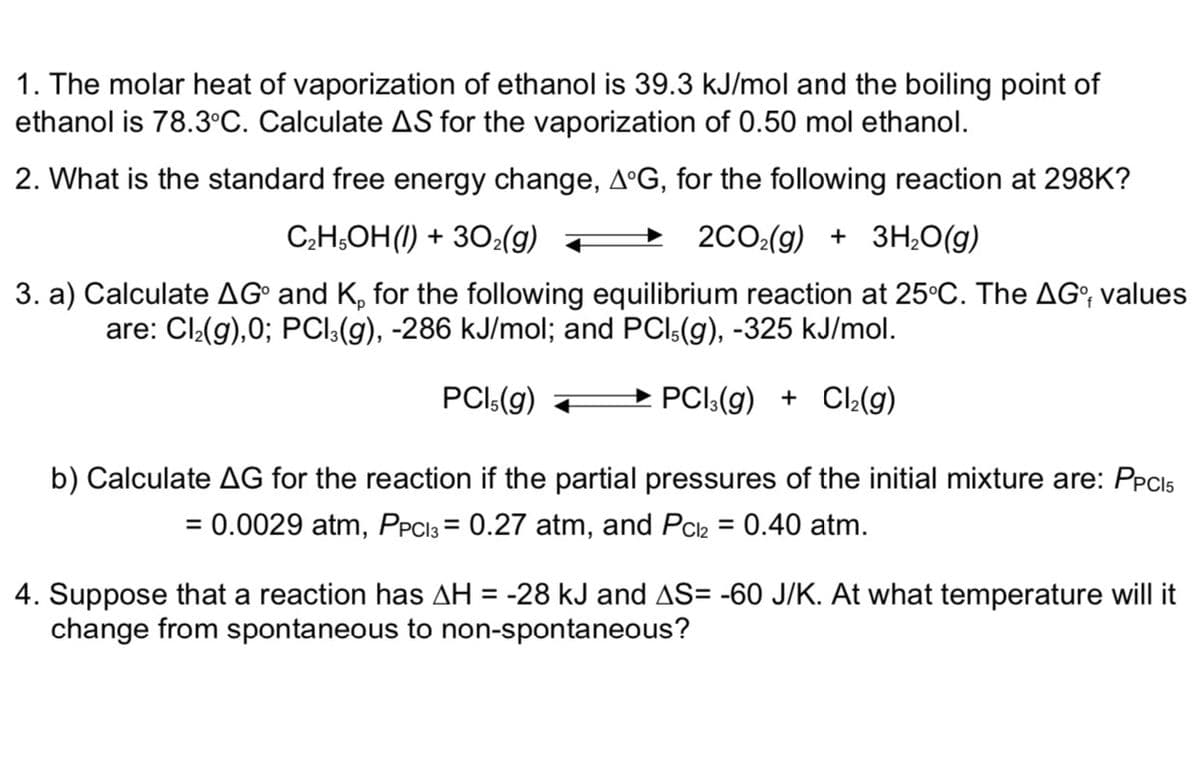
Transcribed Image Text:1. The molar heat of vaporization of ethanol is 39.3 kJ/mol and the boiling point of
ethanol is 78.3°C. Calculate AS for the vaporization of 0.50 mol ethanol.
2. What is the standard free energy change, AºG, for the following reaction at 298K?
C;H,OH(1) + 30:(g) +
2CO:(g) + 3H,0(g)
3. a) Calculate AG° and K, for the following equilibrium reaction at 25°C. The AG; values
are: Cl(g),0; PCI:(g), -286 kJ/mol; and PCI:(g), -325 kJ/mol.
PCI:(g)
→ PCI:(g) + CI:(g)
b) Calculate AG for the reaction if the partial pressures of the initial mixture are: PPCIS
= 0.0029 atm, PPCI3 = 0.27 atm, and Pck = 0.40 atm.
%3D
%3D
4. Suppose that a reaction has AH = -28 kJ and AS= -60 J/K. At what temperature will it
change from spontaneous to non-spontaneous?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning