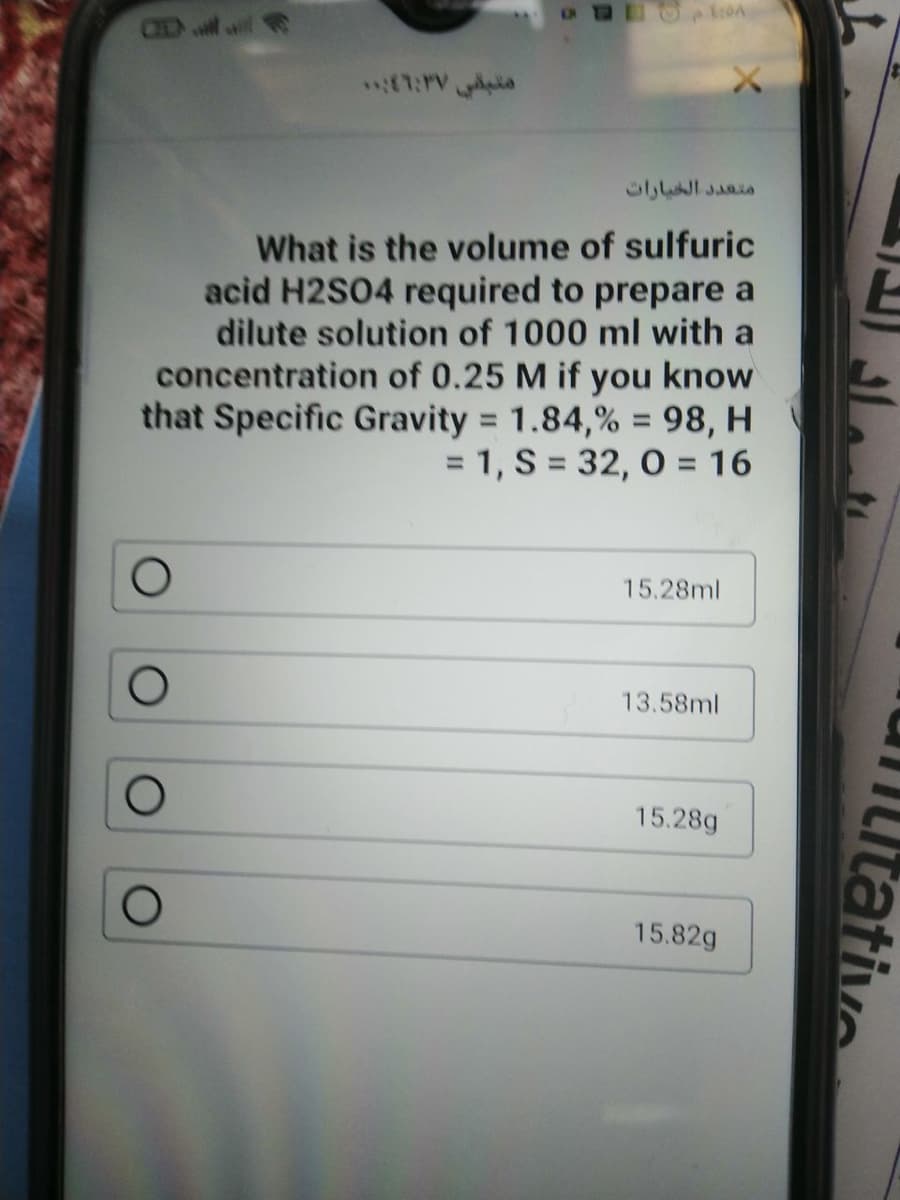
What is the volume of sulfuric acid H2S04 required to prepare a dilute solution of 1000 ml with a concentration of 0.25 M if you know that Specific Gravity = 1.84,% = 98, H = 1, S = 32, 0 = 16 %3D 15.28ml 13.58ml 15.28g 15.82g
What is the volume of sulfuric acid H2S04 required to prepare a dilute solution of 1000 ml with a concentration of 0.25 M if you know that Specific Gravity = 1.84,% = 98, H = 1, S = 32, 0 = 16 %3D 15.28ml 13.58ml 15.28g 15.82g
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.10QAP
Related questions
Question
Transcribed Image Text:CED ll il
What is the volume of sulfuric
acid H2S04 required to prepare a
dilute solution of 1000 ml with a
concentration of 0.25 M if you know
that Specific Gravity = 1.84,% = 98, H
= 1, S = 32, 0 = 16
%3D
%3D
%3D
15.28ml
13.58ml
15.28g
15.82g
Litative
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning