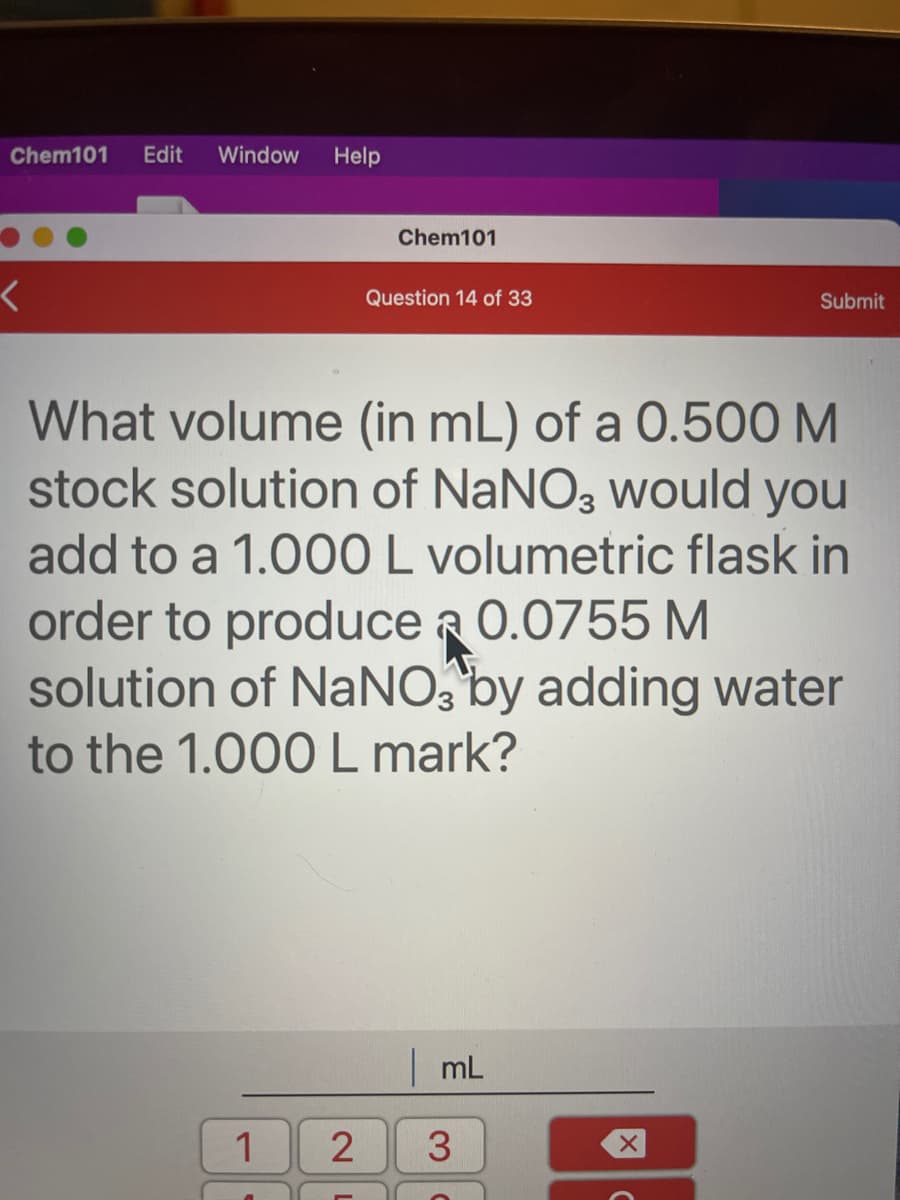
What volume (in mL) of a 0.500 M stock solution of NaNO3 would you add to a 1.000 L volumetric flask in order to produce a 0.0755 M solution of NaNO, by adding water
Q: What volume in milliliters of 0.0130 M Sr(OH)₂ is required to neutralize 60.0 mL of 0.0400 M HBr?
A: Given data contains, Molarity of Sr(OH)₂ is 0.0130 M. Molarity of HBr is 0.0400 M. Volume of HBr is…
Q: What volume of 3.63 M HCIag) is needed to make 350.0 mL of a 0.169 M hydrochloric acid solution?
A:
Q: What mass of KBrKBr (in grams) should you use to make 250.0 mLmL of a 1.10 MM solution of KBrKBr?
A: Given data, Volume of solution is 250 mL =0.25L (1L =1000mL) Molarity of…
Q: A 9.44 g sample of an aqueous solution of hydrobromic acid contains an unknown amount of the acid.…
A: Given that : The mass of the sample = 9.44 g The volume of barium hydroxide = 29.4 mL The molarity…
Q: An aqueous solution of sodium hydroxide is standardized by titration with a 0.142 M solution of…
A:
Q: Analysis of a solution of NaOH showed that 42.67 mL of 0.485 M HNO3 was needed to titrate a 25.00-mL…
A: Given: 42.67mL of 0.485M HNO3 titrated with 25.00mL sample of NaOH. To find: concentration of the…
Q: An aqueous solution of sodium hydroxide is standardized by titration with a 0.117 M solution of…
A: Molarity of perchloric acid = 0.117 M Volume of perchloric acid = 29.6 mL = 0.0296 L Volume of NaOH…
Q: Suppose that you are given a stock solution of 1.14 mol/L ammonium sulfate. What volume of solution…
A:
Q: You have 500 mL of a 0.125 M HCl(aq) solution and you want to dilute it to exactly 0.100 M. How much…
A: The given problem relates to stock dilution, so we can use the below relation to calculate the…
Q: What volume of 0.311 M sulfurous acid solution could be neutralized by 2.00 g aluminum hydroxide?…
A: During the neutralization process, the following reaction takes place: 3H2SO3 + 2Al(OH)3 → Al2(SO3)3…
Q: An aqueous solution of calcium hydroxide is standardized by titration with a 0.113 M solution of…
A:
Q: A mixture of hydrochloric and sulfuric acids is prepared so that it contains 0.235 M HCl and 0.605 M…
A: Given:Molarity of HCl = 0.235 MMolarity of H2SO4 = 0.605 MTotal volume = 273.1 mLMolarity of NaOH =…
Q: In a titration, 25.00 ML of 0.300 M sodium hydroxide solution is exactly neutralized by 37.5 ML of a…
A: Given: Volume of sodium hydroxide, V1 =25.00 mL Concentration of sodium hydroxide, M1 = 0.300 M…
Q: An acid solution is 0.100 M in HCl and 0.200 M in H2SO4. What volume of a 0.150 M KOH solution would…
A: Given: The molarity of HCl is 0.1 M. The molarity of H2SO4 is 0.2 M. The molarity of KOH is 0.15 M.…
Q: A mixture of hydrochloric acid and sulfuric acid is prepared so that it contains 0.635 M and 0.135 M…
A: Answer is explained below.
Q: How many milliliters of an aqueous solution of 0.217 M copper(II) fluoride is needed to obtain 17.1…
A: The molarity of the solution is = 0.127 M The mass of copper(II) fluoride (solute) is = 17.1 g The…
Q: An aqueous solution of potassium hydroxide is standardized by titration with a 0.107 M solution of…
A:
Q: 1.00 L of a solution is prepared by dissolving 125.6 g of NaF in it. If I took 180 mL of that…
A: Given : Mass of NaF = 125.6 g Volume of initial solution = 1.00 L Volume of initial solution used =…
Q: 1.3334 grams of Aluminum chloride was dissolved in distilled water up to 100 mL. What is the molar…
A:
Q: How many ml. of a 0.230 M aqueous solution of ammonium nitrate, NH,NO,, must be taken to obtain 3.37…
A: Mass of salt = 3.37 g Determination of moles of salt - Moles of NH4NO3 =MassMolar mass=3.37…
Q: A 16.2-g sample of HF is dissolved in water to give 3.0 × 102 mL of solution. What is the…
A:
Q: What volume of each of the following bases will react completely with 52.00 mL of 0.380 M HCl?…
A:
Q: The
A: Molarity is defined as total number of solute present in 1000 ml of solution . For neutralisation…
Q: The molarity of an aqueous solution of barium hydroxide is determined by titration against a 0.292 M…
A: Given:- M1=HCl=0.292M, V1=19.9 mL n1=Basicity of acid=1 M2=BaOH2=? V2=38.3 mL n2=Acidity of…
Q: A student removes 25 mL of 3.0 M NaOH and dilutes it to final volume of 350 mL. What is the…
A: Given :- initial concentration of solution = 3.0 M initial volume of solution = 25 mL final volum…
Q: A mixture of hydrochloric and sulfuric acids is prepared so that it contains 0.575 M HCl and 0.145 M…
A: The moles of HCl and sulfuric acid is calculated.
Q: An aqueous solution of potassium hydroxide is standardized by titration with a 0.148 M solution of…
A: EXPLANATION: Reaction of acid and base to form salt and water is called neutralisation…
Q: If a 57.0 mL sample of nitric acid is titrated with .375 M calcium hydroxide, and 23.0 mL are…
A: The reaction taking place is 2HNO3 + Ca(OH)2 ----> Ca(NO3)2 + 2H2O => for 2 moles of HNO3 we…
Q: A student weighs out a 15.8 g sample of aluminum bromide, transfers it to a 300 mL volumetric flask,…
A: AlBr3→Molar mass of AlBr3 is 266.699 gmol-1 . So 1 g AlBr3 has (1÷ 266.699) gmol-1 So 15.8 g of…
Q: 4. You are given 150 ml of a 0.1 M NAOH solution. What volume of a 1M HCI solution would you need to…
A: Given :- molarity of NaOH solution = 0.1 M Volume of NaOH solution =150 mL Molarity of HCl…
Q: An aqueous solution of potassium hydroxide is standardized by titration with a0.174 M solution of…
A: Given that Aqueous solution of Potassium hydroxide is standardized by titration with a 0.174 M…
Q: A student pipets 13 mL of a 0.0115 M solution of CaCl2 into a 100.00 mL volumetric flask and dilutes…
A: Given,
Q: A student weighs out a 3.79 g sample of potassium nitrate, transfers it to a 250 mL volumetric…
A: Given, mass of KNO3 = 3.79 g. Volume of the solution = 250 mL = 250 mL×1 L1000 mL = 0.25 L. We have…
Q: What volume in milliliters of 9.76 M hydrochloric acid solution should be used to prepare 2.00 L of…
A: In the dilution process, the relationship between initial and final concentrations and volumes of…
Q: A very strong nitric acid (HNO3=63.02 g/mol) solution is available as a 33.4 m solution. Given the…
A: Given: Molality = 33.4 m Density of solution = 1.33 g/mL Molar mass of solute (HNO3) = 63.02 g/mL…
Q: if 34.1g of Nal (MM= 149.89g/mol) are added to a 500.0mL volumetric flask, and water is added to…
A:
Q: If 12.5 mL of a 0.20 M NaOH solution is diluted to 52.0mL, what is the concentration of the…
A: For dilution M.V = constant
Q: A stock solution of lithium sulfate has a concentration of 5.5 M. If 88.3 mL of stock solution is…
A: Given :- Initial concentration (M1) = 5.5 M initial volume (V1) = 88.3 mL Final volume (V2) =…
Q: if 23.1g of NaOH ( MM= 40.00g/mol) are added to a 500ML volumetric flask, and water is added to fill…
A: Given : Mass of NaOH = 23.1 g Molar mass of NaOH = 40.00 g.mol-1 Volume of solution = 500 mL To…
Q: You have 505 mL of a 0.125 M HCl solution and you want to dilute it to exactly 0.100 M. How much…
A: Concentration(c) = no of moles/volume No. of moles = concentration(c) * volume(v) If dilution…
Q: An acid solution is 0.100 M in HCl and 0.200 M in H2SO4. What volume of a 0.150 M KOH solution would…
A:
Q: Suppose that you are given a stock solution of 1.14 mol/L ammonium sulfate. What volume of solution…
A:
Q: A l1.5 g sample of an aqueous solution of hydroiodic acid contains an unknown amount of the acid. If…
A: Given, Mass of Mixture = 11.5 g Molarity of NaOH = 0.898 M = 0.898 mol/L Volume of NaOH = 12.2 mL =…
Q: How many mL of a 0.185 M aqueous solution of cobalt(Il) iodide, Col2, must be taken to obtain 20.5…
A: Cobalt (ll) Iodide ( CoI2 ) Molarity = 0.185 M = 0.185 mol/L Mass of CoI2 = 20.5 g We know ;…
Q: How many molecules of hydrosulfuric acid (H₂S) could be reacted by 22.00 ml of 0.135 M sodium…
A: The reaction between H2S and NaOH is given by, H2S + 2NaOH - - - - > Na2S + 2H2O In question they…
Q: If a 8. 2 M of solution of KNO3 reaches a volume of 450 ml. what is the moles of solute needed?
A:
Q: What volume of 0.687 M perchloric acid solution (in mL) is needed to neutralize 50.00 mL of 0.0347 M…
A:
Q: An aqueous solution of sodium hydroxide is standardized by titration with a 0.107 M solution of…
A: Given Molarity of HI ( M1 ) = 0.107 M Volume of the acid ( V1 ) = 24.7 mL Volume of base ( V2 ) =…
Q: A 8.84 g sample of an aqueous solution of hydrobromic acid contains an unknown amount of the acid.…
A:
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

- 5. Given 200cm3 of pure water at 40C, what volume of a solution of hydrochloric acid whose density is 1.175g/cm3 and containing 344.4% HCl by weight, could be prepared?50g of nitrogen (N2) has a volume of ___ liters at STP. Show the complete solution a. V=49.0L b. V=40.0L c. V=44.9L d. V=47.70LThe concentration of mercury in a polluted lakeis 4.5 ppb (part per billion), and the density of water is 1.0 g/mL. (a) Calculatethe concentration of mercury: (i)in g/L (microgram per liter), and (ii) in mol/L.(b)If the lake has surface area of 1.3km2and an average depth of 16m, how many kilograms of mercury are present in the lake?
- What will be the final volume of 100. ml of N2 at -20.0º C if you heated it to 10.0º C?The density of concentrated ammonia, which is 28.0% w/w NH3 is 0.899 g/mL. What volume of this reagent should you dilute yo 1.0x10^3 mL to make a solution that is 0.036M in NH3Rx Sodium chloride solution . . . . . . . . . . . . . . 0.9% w/v Purified water, q.s. . . . . . . . . . . . . . . . . . . . 1000 mL Sig. For irrigation. The volume of a 1:10 w/v stock solution of sodium chloride that should be used in compounding 800 mL of the above prescription is _____ mL
- What is the boiling point of the automobile radiator fluid prepared by mixing 1.11 L of ethylene glycol (HOCH2CH2OH, density = 1.114 g/mL) with 1.06 L of water (density = 1.000 g/mL)?The Kb of water is 0.520°C/m.The active ingredient of Benadryl© Chesty Forte Cough Liquid is Guaiphenesin anexpectorant drug used to assist the expectoration ('bringing up') of phlegm from theairways in acute respiratory tract infections. A 200.0 mL bottle of Benadryl© ChestyForte Cough contains 4.00 g of Guaiphenesin (C10H14O4). i. If the recommended dose for a child is 150 mg of Guaiphenesin. What volume of thecough liquid should be administered? ii. If the recommended dose for an adult is 350 mg of Guaiphenesin. How many molesof Guaiphenesin does this equal?The active ingredient of Benadryl© Chesty Forte Cough Liquid is Guaiphenesin anexpectorant drug used to assist the expectoration ('bringing up') of phlegm from theairways in acute respiratory tract infections. A 200.0 mL bottle of Benadryl© ChestyForte Cough contains 4.00 g of Guaiphenesin (C10H14O4). i. If the recommended dose for a child is 150 mg of Guaiphenesin. What volume of thecough liquid should be administered? ii. If the recommended dose for an adult is 350 mg of Guaiphenesin. How many molesof Guaiphenesin does this equal? (answer to 2 significant figures)
- The active ingredient of Benadryl© Chesty Forte Cough Liquid is Guaiphenesin anexpectorant drug used to assist the expectoration ('bringing up') of phlegm from theairways in acute respiratory tract infections. A 200.0 mL bottle of Benadryl© ChestyForte Cough contains 4.00 g of Guaiphenesin (C10H14O4). i. If the recommended dose for a child is 150 mg of Guaiphenesin. What volume of thecough liquid should be administered?A river pollutant is found to have a concentration of 0.0031 % m/v. If the density of the river water is 1.183 g/ml, what is the concentration of the pollutant in ppm? Proper rounding of your answer is required.2) A patient visits your pharmacy with a prescription for 200g Betnovate (betamethasone valerate) 0.1% w/w cream . The specials department has a Betnovate 2.5% w/w cream stock concentration.How much diluent needs to be added to the Betnovate 2.5% w/w cream in grams to produce the Betnovate 0.1% w/w cream for the prescription?State your answer to the nearest whole number.unit- g



