What volume (mL) of 0.025 M HNO3 is necessary to titrate 5.2 mL of 0.046 M Ca(OH), solution to the endpoint? Please give the answer to 1 decimal place.
What volume (mL) of 0.025 M HNO3 is necessary to titrate 5.2 mL of 0.046 M Ca(OH), solution to the endpoint? Please give the answer to 1 decimal place.
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.15QAP
Related questions
Question
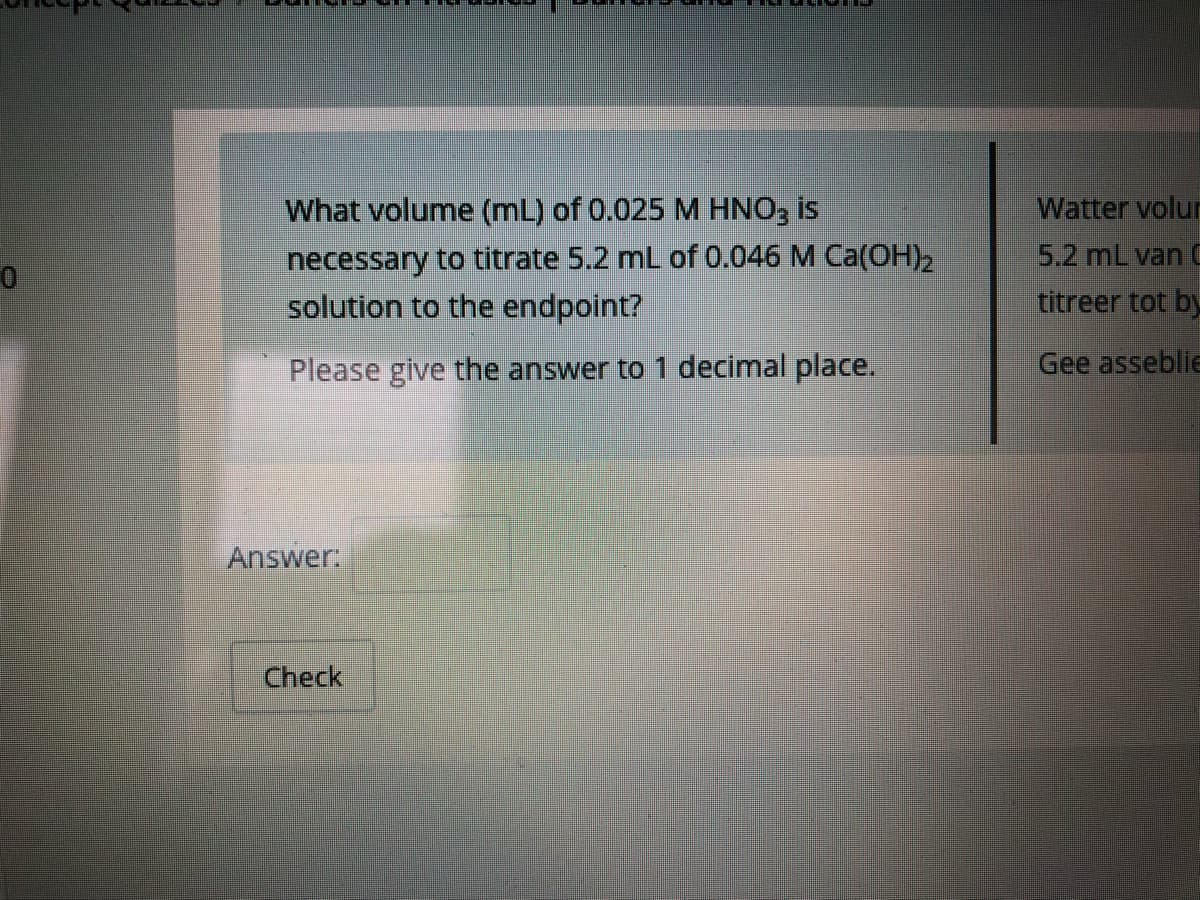
Transcribed Image Text:What volume (mL) of 0.025 M HNO, is
Watter volur
necessary to titrate 5.2 mL of 0.046 M Ca(OH)2
solution to the endpoint?
5.2 mL van C
titreer tot by
Please give the answer to 1 decimal place.
Gee asseblie
Answer:
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning