What volume of hydrogen gas is prođuced when 1.04 mol of iron reacts completely according to the following reaction at 0 C and 1 atm? iron (s)hydrochloric acid ( aq)-> iron(II) chloride ( aq)hydrogen (g) liters hydrogen gas
What volume of hydrogen gas is prođuced when 1.04 mol of iron reacts completely according to the following reaction at 0 C and 1 atm? iron (s)hydrochloric acid ( aq)-> iron(II) chloride ( aq)hydrogen (g) liters hydrogen gas
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.113P: 5-113 Ammonia and gaseous hydrogen chloride react to form ammonium chloride according to the...
Related questions
Question
please see attached
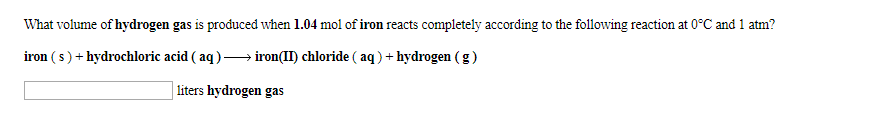
Transcribed Image Text:What volume of hydrogen gas is prođuced when 1.04 mol of iron reacts completely according to the following reaction at 0 C and 1 atm?
iron (s)hydrochloric acid ( aq)-> iron(II) chloride ( aq)hydrogen (g)
liters hydrogen gas
Expert Solution
Step 1
Given reaction:
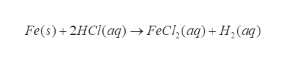
Step 2
Ideal gas equation gives the collision between the molecules and atoms. The ideal gas equation:

Step 3
- Rearranging the equation and substituting the respective values:
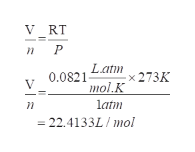
Step by step
Solved in 6 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning