Determine the following values for a saturated aqueous NaOH solution at 20 °C to the indicated number of decimal places (i.e. "d.p."). Enter the appropriate answers into Canvas. Note: Avoid aggressive rounding. Some of the numbers you calculate early will be used later. Multi- step calculations only get rounded at the very end. Question Name Unit d.p. Value msolu g msolv g 0 3 msoln 2.
Determine the following values for a saturated aqueous NaOH solution at 20 °C to the indicated number of decimal places (i.e. "d.p."). Enter the appropriate answers into Canvas. Note: Avoid aggressive rounding. Some of the numbers you calculate early will be used later. Multi- step calculations only get rounded at the very end. Question Name Unit d.p. Value msolu g msolv g 0 3 msoln 2.
Chapter12: Spectrochemical Methods
Section: Chapter Questions
Problem 7P
Related questions
Question
Transcribed Image Text:Electroly
Concentr X
Chapter
Partial P
henrys la x
My Q
'index.html
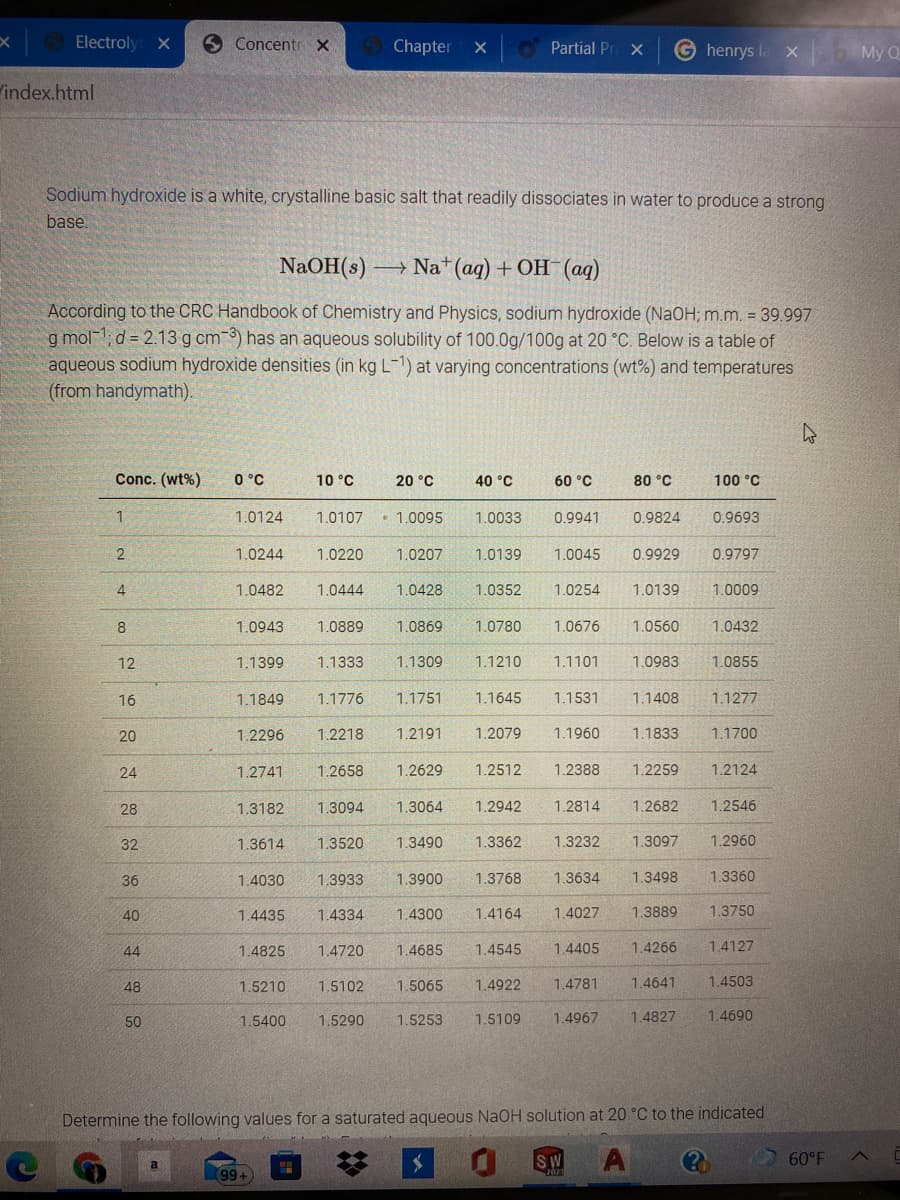
Sodium hydroxide is a white, crystalline basic salt that readily dissociates in water to produce a strong
base
NaOH(s) –
- Na* (aq) + OH (aq)
According to the CRC Handbook of Chemistry and Physics, sodium hydroxide (NaOH; m.m. = 39.997
g mol-1; d = 2.13 g cm 3) has an aqueous solubility of 100.0g/100g at 20 °C. Below is a table of
aqueous sodium hydroxide densities (in kg L-1) at varying concentrations (wt%) and temperatures
(from handymath).
Conc. (wt%)
0 °C
10 °C
20 °C
40 °C
60 °C
80 °C
100 °C
1.0124
1.0107
• 1.0095
1.0033
0.9941
0.9824
0.9693
2
1.0244
1.0220
1.0207
1.0139
1.0045
0.9929
0.9797
4.
1.0482
1.0444
1.0428
1.0352
1.0254
1.0139
1.0009
8
1.0943
1.0889
1.0869
1.0780
1.0676
1.0560
1.0432
12
1.1399
1.1333
1.1309
1.1210
1.1101
1.0983
1.0855
16
1.1849
1.1776
1.1751
1.1645
1.1531
1.1408
1.1277
20
1.2296
1.2218
1.2191
1.2079
1.1960
1.1833
1.1700
24
1.2741
1.2658
1.2629
1.2512
1.2388
1.2259
1.2124
28
1.3182
1.3094
1.3064
1.2942
1.2814
1.2682
1.2546
32
1.3614
1.3520
1.3490
1.3362
1.3232
1.3097
1.2960
36
1.4030
1.3933
1.3900
1.3768
1.3634
1.3498
1.3360
40
1.4435
1.4334
1.4300
1.4164
1.4027
1.3889
1.3750
44
1.4825
1.4720
1.4685
1.4545
1.4405
1.4266
1.4127
48
1.5210
1.5102
1.5065
1.4922
1.4781
1.4641
1.4503
50
1.5400
1.5290
1.5253
1.5109
1.4967
1.4827
1.4690
Determine the following values for a saturated aqueous NaOH solution at 20 °C to the indicated
SW
60°F
(99
Transcribed Image Text:Electroly
Concentr
Chapter
Partial Pr
G henrys la
/index.html
50
1.5400
1.5290
1.5253
1.5109
1.4967
1.4827
1.4690
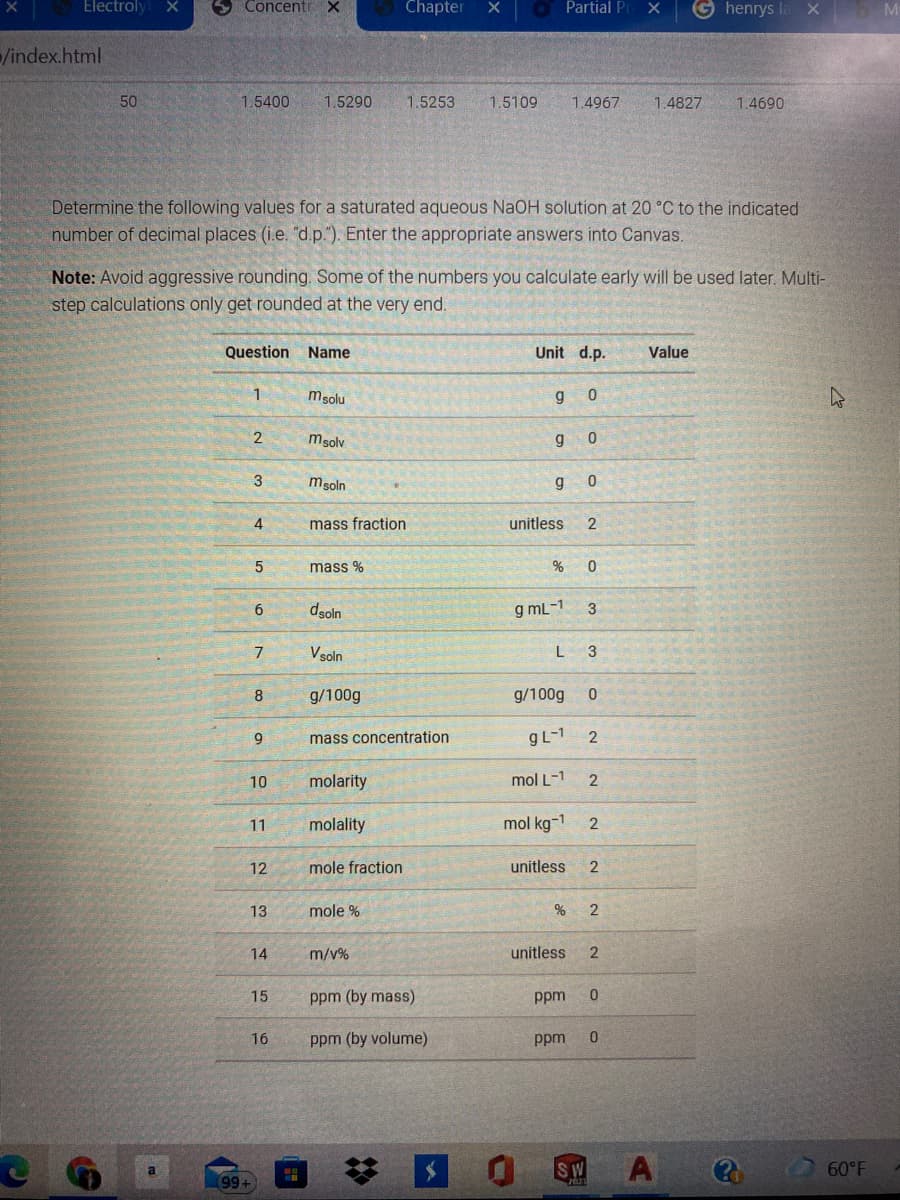
Determine the following values for a saturated aqueous NaOH solution at 20 °C to the indicated
number of decimal places (i.e. "d.p."). Enter the appropriate answers into Canvas.
Note: Avoid aggressive rounding. Some of the numbers you calculate early will be used later. Multi-
step calculations only get rounded at the very end.
Question Name
Unit d.p.
Value
1
msolu
g
msolv
g
3
msoln
g
4
mass fraction
unitless
2
mass %
dsoln
g mL-1
6
3
Vsoln
3
8
g/100g
g/100g
9.
mass concentration
gL-1
2
10
molarity
mol L-1
11
molality
mol kg-1
12
mole fraction
unitless
2
13
mole %
14
m/v%
unitless
15
ppm (by mass)
ppm
16
ppm (by volume)
ppm
SW
60°F
99+
%23
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Could the remaining questions be answered as well?
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning