When a nucleus undergoes radioactive decay a daughter nucleus is formed. The daughter nucleus is lighter and more stable than the nucleus that decayed. When an unstable element emits an a ray, the phenomenon is known as a decay. Similarly, when an unstable element emits a 3 ray, the phenomenon is known as 3 decay, and when an unstable element emits a y ray, the phenomenon is known as y decay. The element that emits radiation is known as the parent element, and the resulting element is known as the daughter element. You can consider the following rules when identifying the type of radioactive decay present in an element. 1. During a decay, the mass number of the parent element decreases by 4, and the atomic number decreases by 2. 2. When an atom emits a 3 ray, the mass number does not change, and the atomic number of the parent element increases by 1. This occurs because a Bray is composed of only high-speed electrons, and electrons have negligible mass and carry a negative charge. 3. During y decay, neither the atomic number nor the mass number changes, because a y ray is an electromagnetic radiation that has neither mass nor charge. Part A Identify the product of radioactive decay Identify the product of radioactive decay and classify the given nuclear reactions accordingly. Drag the appropriate items to their respective bins. View Available Hint(s) a decay 23Ra-2Rn+? 24Pb-2Bi+? 22Pu-22U+? 1N 180+? Ni-Ni+? decay y decay Reset Help
When a nucleus undergoes radioactive decay a daughter nucleus is formed. The daughter nucleus is lighter and more stable than the nucleus that decayed. When an unstable element emits an a ray, the phenomenon is known as a decay. Similarly, when an unstable element emits a 3 ray, the phenomenon is known as 3 decay, and when an unstable element emits a y ray, the phenomenon is known as y decay. The element that emits radiation is known as the parent element, and the resulting element is known as the daughter element. You can consider the following rules when identifying the type of radioactive decay present in an element. 1. During a decay, the mass number of the parent element decreases by 4, and the atomic number decreases by 2. 2. When an atom emits a 3 ray, the mass number does not change, and the atomic number of the parent element increases by 1. This occurs because a Bray is composed of only high-speed electrons, and electrons have negligible mass and carry a negative charge. 3. During y decay, neither the atomic number nor the mass number changes, because a y ray is an electromagnetic radiation that has neither mass nor charge. Part A Identify the product of radioactive decay Identify the product of radioactive decay and classify the given nuclear reactions accordingly. Drag the appropriate items to their respective bins. View Available Hint(s) a decay 23Ra-2Rn+? 24Pb-2Bi+? 22Pu-22U+? 1N 180+? Ni-Ni+? decay y decay Reset Help
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter14: Nuclear Chemistry
Section: Chapter Questions
Problem 14.36PAE
Related questions
Question
100%
14) please see picture
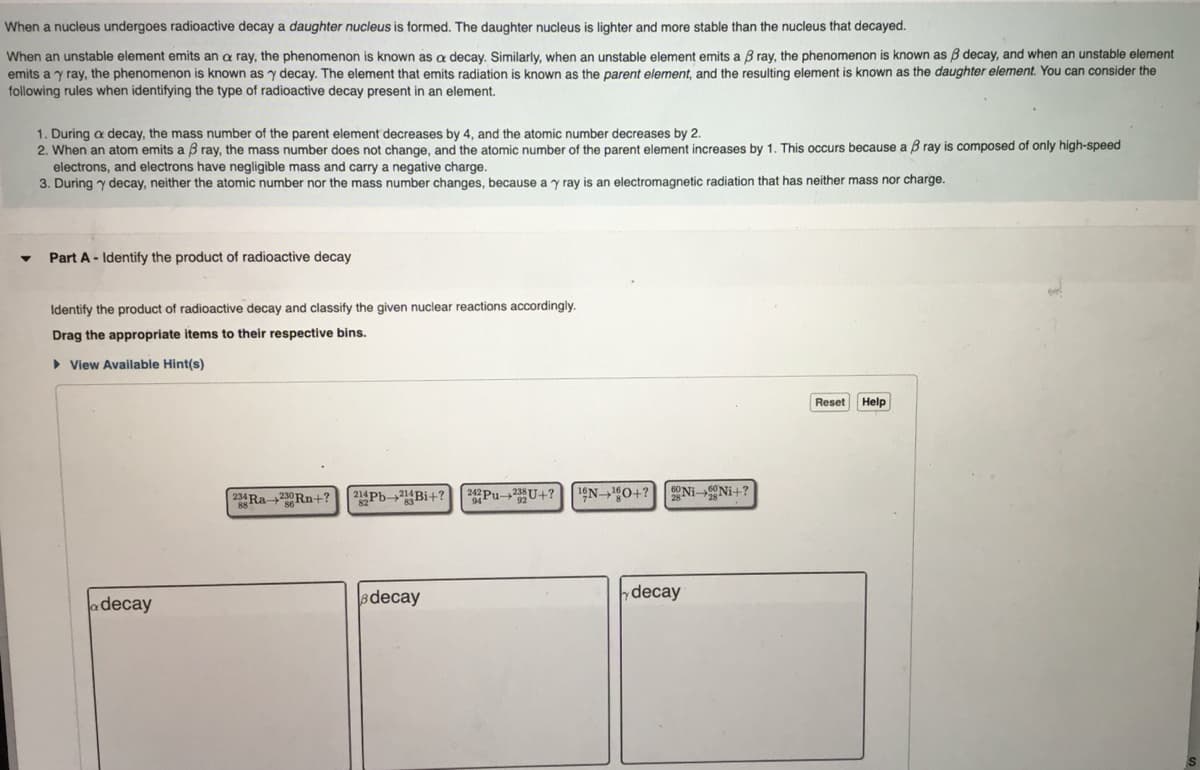
Transcribed Image Text:When a nucleus undergoes radioactive decay a daughter nucleus is formed. The daughter nucleus is lighter and more stable than the nucleus that decayed.
When an unstable element emits an a ray, the phenomenon is known as a decay. Similarly, when an unstable element emits a 3 ray, the phenomenon is known as 3 decay, and when an unstable element
emits a y ray, the phenomenon is known as y decay. The element that emits radiation is known as the parent element, and the resulting element is known as the daughter element. You can consider the
following rules when identifying the type of radioactive decay present in an element.
1. During a decay, the mass number of the parent element decreases by 4, and the atomic number decreases by 2.
2. When an atom emits a ß ray, the mass number does not change, and the atomic number of the parent element increases by 1. This occurs because a ß ray is composed of only high-speed
electrons, and electrons have negligible mass and carry a negative charge.
3. During y decay, neither the atomic number nor the mass number changes, because a y ray is an electromagnetic radiation that has neither mass nor charge.
Part A Identify the product of radioactive decay
Identify the product of radioactive decay and classify the given nuclear reactions accordingly.
Drag the appropriate items to their respective bins.
View Available Hint(s)
a decay
23Ra 23Rn+? 214Pb-2Bi+? 22Pu-23U+? 1N 180+? Ni-Ni+?
decay
y decay
Reset Help
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning