When marking the sample line on a TLC plate, why is it inadvisable to use a ball-point pen? 5-31. A series of dyes is separated by TLC. The data are given below. Calculate the Rf value for each dye. 5-32. Why is it important not to let the level of the elution solvent in a packed chromatographic column drop below the top of the solid-phase adsorbent? 5-33. What are some advantages of using column chromatography to purify reaction products in the microscale laboratory? 5-34. Discuss the similarities and dissimilarities of TLC, paper, and column chromatography. 5-35. Discuss the similarities and dissimilarities of HPLC and gas chromatography. 5-36. (a) What are the main advantages of using flash chromatography? (b) How can TLC be used in connection with flash chromatography? 5-37. Using the information presented on the right, please identify and explain which spot has an Rf value of 0.5.
(please answer all questions)
5-30. When marking the sample line on a TLC plate, why is it inadvisable to use a ball-point pen?
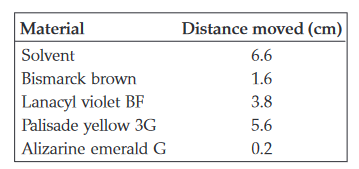
5-31. A series of dyes is separated by TLC. The data are given below. Calculate the Rf value for each dye.
5-32. Why is it important not to let the level of the elution solvent in a packed chromatographic column drop below the
top of the solid-phase adsorbent?
5-33. What are some advantages of using column chromatography to purify reaction products in the microscale
laboratory?
5-34. Discuss the similarities and dissimilarities of TLC, paper, and column chromatography.
5-35. Discuss the similarities and dissimilarities of HPLC and gas chromatography.
5-36. (a) What are the main advantages of using flash chromatography?
(b) How can TLC be used in connection with flash chromatography?
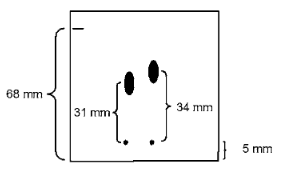
5-37. Using the information presented on the right, please identify and explain
which spot has an Rf value of 0.5.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images






