When the following skeletal equation is balanced under acidic conditions, what are the coefficients of the species shown? HNO2 + |ClO3 - NO3¯+ CIO2 Water appears in the balanced equation as a |(reactant, product, neither) with a coefficient of (Enter 0 for neither.) Which species is the oxidizing agent?
When the following skeletal equation is balanced under acidic conditions, what are the coefficients of the species shown? HNO2 + |ClO3 - NO3¯+ CIO2 Water appears in the balanced equation as a |(reactant, product, neither) with a coefficient of (Enter 0 for neither.) Which species is the oxidizing agent?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 25QAP: For the following half-reactions, answer these questions....
Related questions
Question
100%
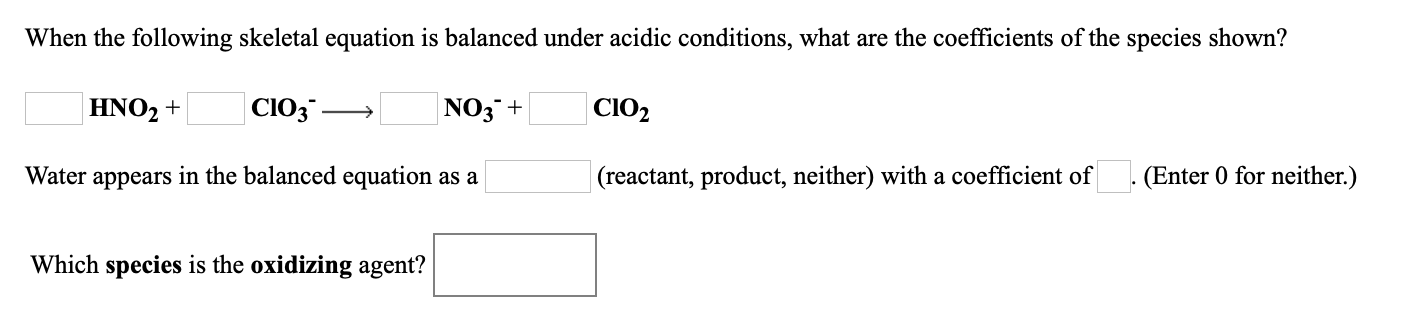
Transcribed Image Text:When the following skeletal equation is balanced under acidic conditions, what are the coefficients of the species shown?
HNO2 +
|ClO3 -
NO3¯+
CIO2
Water appears in the balanced equation as a
|(reactant, product, neither) with a coefficient of
(Enter 0 for neither.)
Which species is the oxidizing agent?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning