When the ionic compound Cs/O4 is dissolved in water, which species will react with water to a considerable extent? You can assume the compound is completely soluble in water. OOOO anion cation both neither J
When the ionic compound Cs/O4 is dissolved in water, which species will react with water to a considerable extent? You can assume the compound is completely soluble in water. OOOO anion cation both neither J
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter18: Representative Metals, Metalloids, And Nonmetals
Section: Chapter Questions
Problem 71E: What volume of 0.200 M NaOH is necessary to neutralize the solution produced by dissolving 2.00 g of...
Related questions
Question
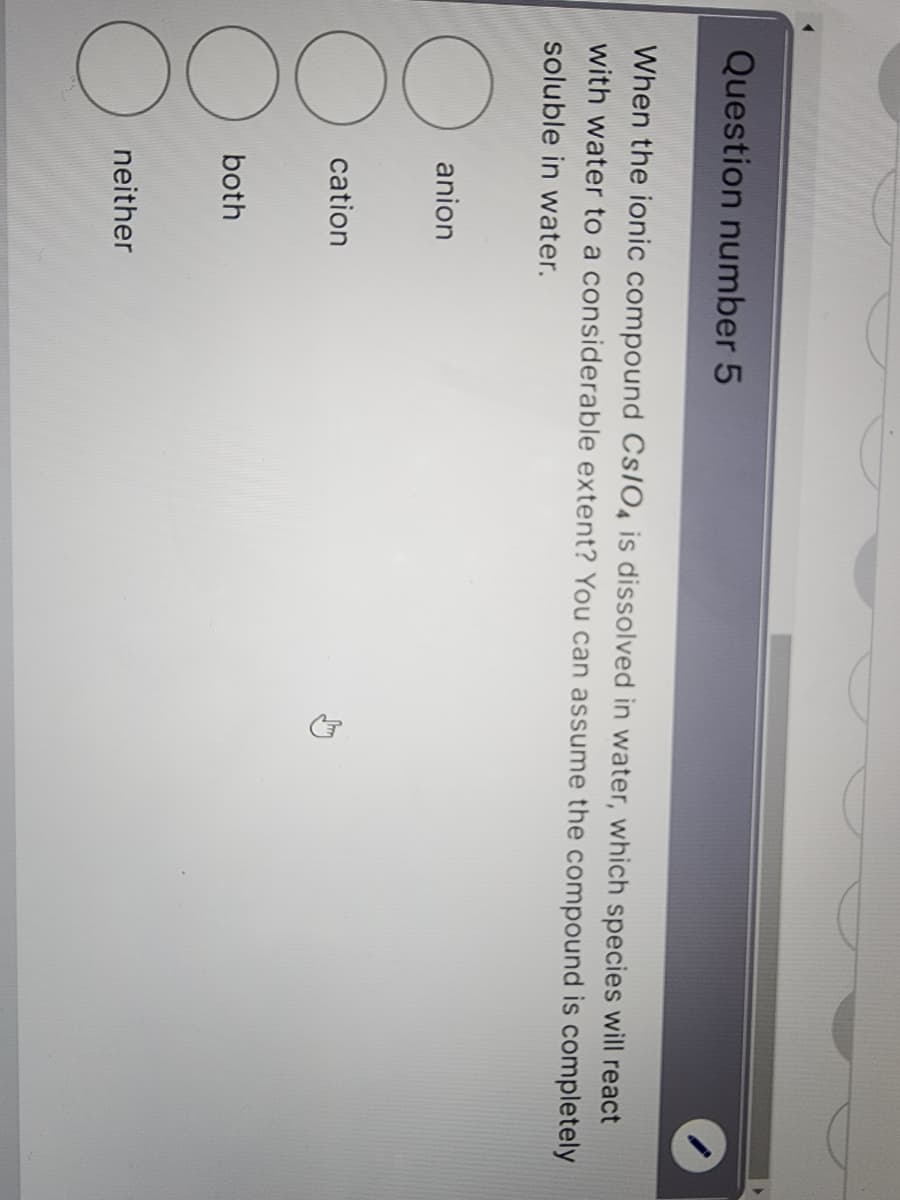
Transcribed Image Text:Question number 5
When the ionic compound CS/O4 is dissolved in water, which species will react
with water to a considerable extent? You can assume the compound is completely
soluble in water.
O
OOO
anion
cation
both
neither
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax