Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter13: Structure And Shape
Section: Chapter Questions
Problem 40E
Related questions
Question
Plz help
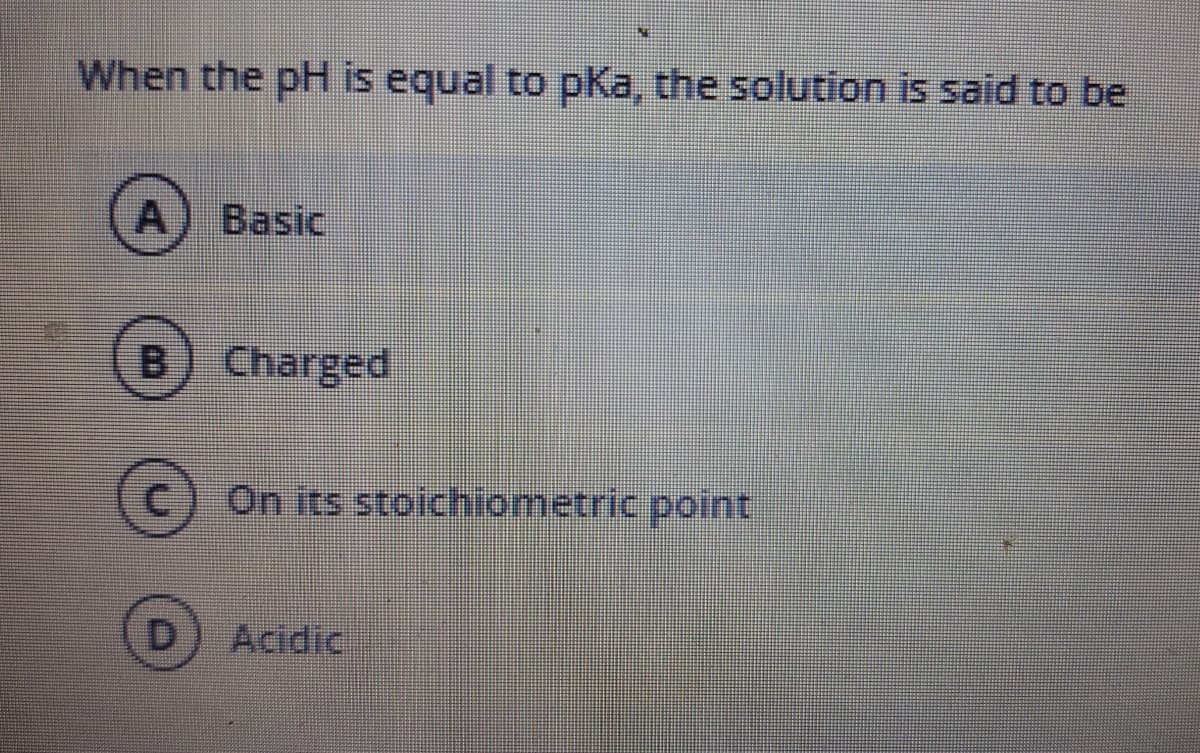
Transcribed Image Text:When the pH is equal to pKa, the solution is said to be
A) Basic
[B
Charged
C.
On its stoichiometric point
Acidic

Transcribed Image Text:Water can both act as acid and base because of its tendency to
Donate and accept a proton to form the hydroxide ion and hydronium ion
Donate and accept the lone pair of electrons in oxygen
Form a covalent and hydrogen bond from the lone pair of electrons in oxygen
Form an ionic bond with its partial positive hydrogen and partial negative oxygen
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning